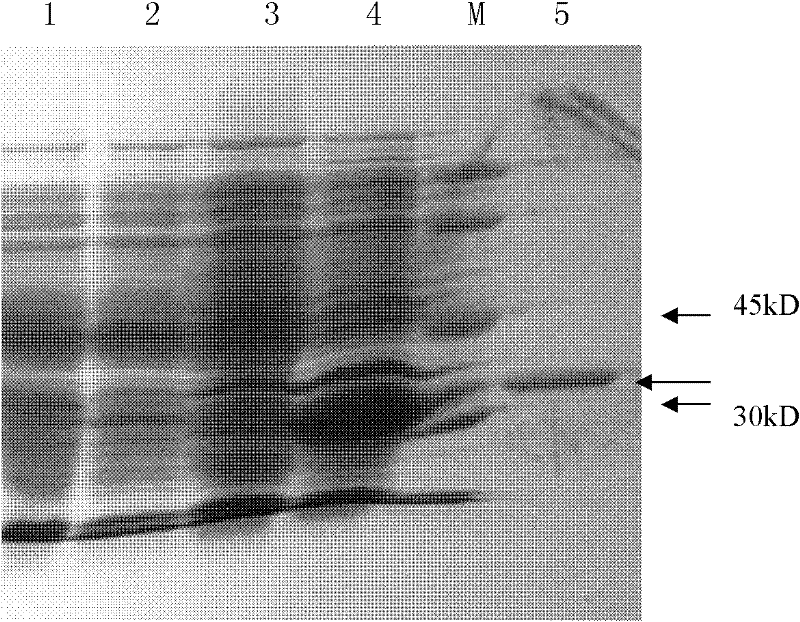
Heat-resisting N-acyl homoserine lactonase AiiA-AIO6 with high specific activity as well as coding gene and application thereof
An acyl homoserine lactone, coding technology, applied in DNA/RNA fragments, applications, genetic engineering, etc., can solve the problems of limited application value, poor heat resistance, low specific activity, etc., and achieve good degradation of various substrates. Ability, strong protease resistance, high specific activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
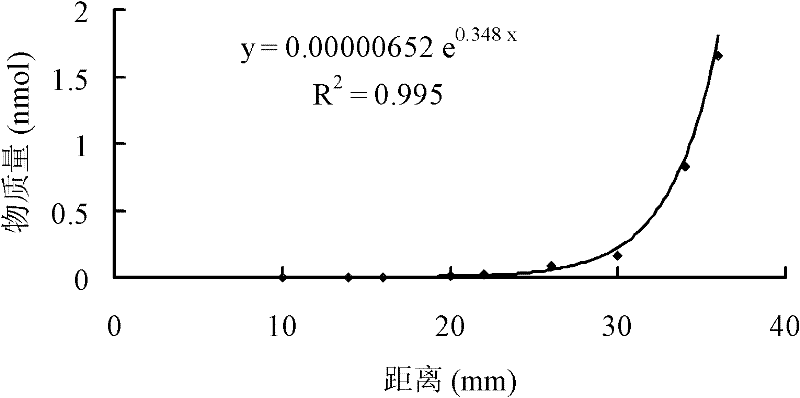
Image
Examples
Embodiment 1
[0058] Embodiment 1, the separation and identification of Pallidum pallidus AIO6
[0059] 1. Isolation of bacteria
[0060] Soil samples were resuspended in sterile water at a ratio of 1:10 (soil:water) and diluted to 10 with sterile water. -8 Afterwards, spread on LB plates (with 5 μM C6-HSL and 3-oxo-C6-HSL), and after culturing at 25°C for 5 days, the colonies were picked into liquid LB and cultured at 25°C for 3 days, centrifuged at 10,000rpm for 1min to collect the cells, and weighed Suspended in 0.1 mol / L PBS buffer (pH 8.0), ultrasonicated and centrifuged at 12000 rpm for 5 min, the supernatant was collected to detect N-acyl homoserine lactonase activity.
[0061] A strain with enzyme activity was obtained, and the strain was named AIO6.
[0062] 2. Identification
[0063] The 16S rDNA of strain AIO6 was amplified and then sequenced, and the sequencing results were compared with the nucleotide sequence in the Genbank database, which proved that the strain AIO6 was Oc...
Embodiment 2
[0074] Embodiment 2, the preparation of recombinant bacteria
[0075] 1. Construction of recombinant expression vector
[0076] 1. Synthesize the DNA (AiiA-AIO6 gene) shown in sequence 2 of the sequence listing.
[0077] 2. Using the DNA synthesized in step 1 as a template, perform PCR amplification with a primer pair composed of A6-F and A6-R (the target sequence is shown in sequence 3 in the sequence listing) to obtain a PCR amplification product.
[0078] A6-F: 5′-CG GAATTC AAATCCCATGAAATCGAGACCAGTC-3' (the underlined part is the EcoR I restriction site);
[0079] A6-R: 5′-TTAA GCGGCCGC TCAGGCCGTGCAGTCG-3' (the underlined part is the Not I restriction site).
[0080] 2. Digest the PCR amplified product of step 1 with restriction endonucleases EcoR I and Not I, and recover the digested product.
[0081] 3. Digest the vector pET-28a(+) with restriction endonucleases EcoR I and Not I to recover the vector backbone (about 5.4kp).
[0082] 4. Ligate the digested product ...
Embodiment 3
[0121] Embodiment 3, AiiA-AIO6 protein as the enzymatic property of N-acyl homoserine lactonase
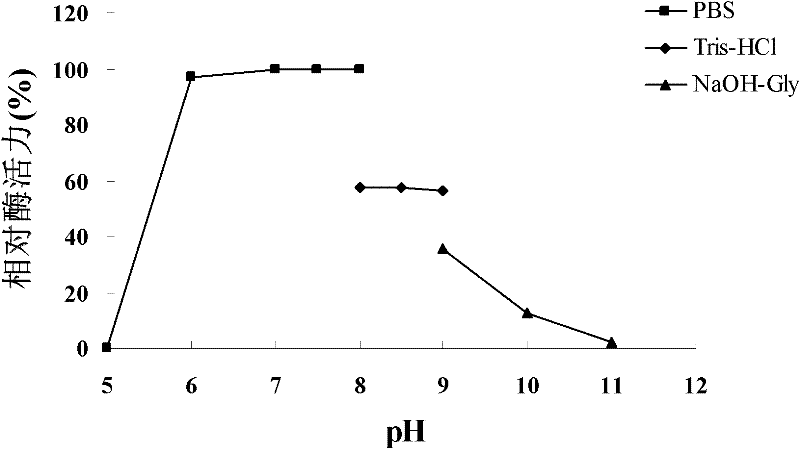
[0122] 1. Optimal pH
[0123] The AiiA-AIO6 protein solution prepared in Example 2 is prepared into a 0.02mg / mL solution (as the solution to be tested) with 0.1mol / L PBS buffer (pH8.0) to carry out the activity of N-acyl homoserine lactonase Detection (in the activity detection, except the buffer solution, it is the same as Step 6 of Example 2 and 3).
[0124] Use the following buffers:
[0125] 0.1mol / L McIlvaine buffer solution of pH 4.0, 5.0, 6.0;
[0126] 0.1mol / L PBS buffer solution with pH 5.0, 6.0, 7.0, 7.5, 8.0;
[0127] Tris-HCl buffer at pH 8.0, 8.5, 9.0;
[0128] 0.1 mol / L glycine-NaOH buffer solution (NaOH-Gly) with pH 9.0, 10.0, 11.0.
[0129] When 0.1mol / L PBS buffer solution (pH8.0) is used, the enzyme activity of the solution to be tested is recorded as 100% (enzyme activity value is 877.04U / mL). For relative enzyme activities using various buffers, see ima...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com