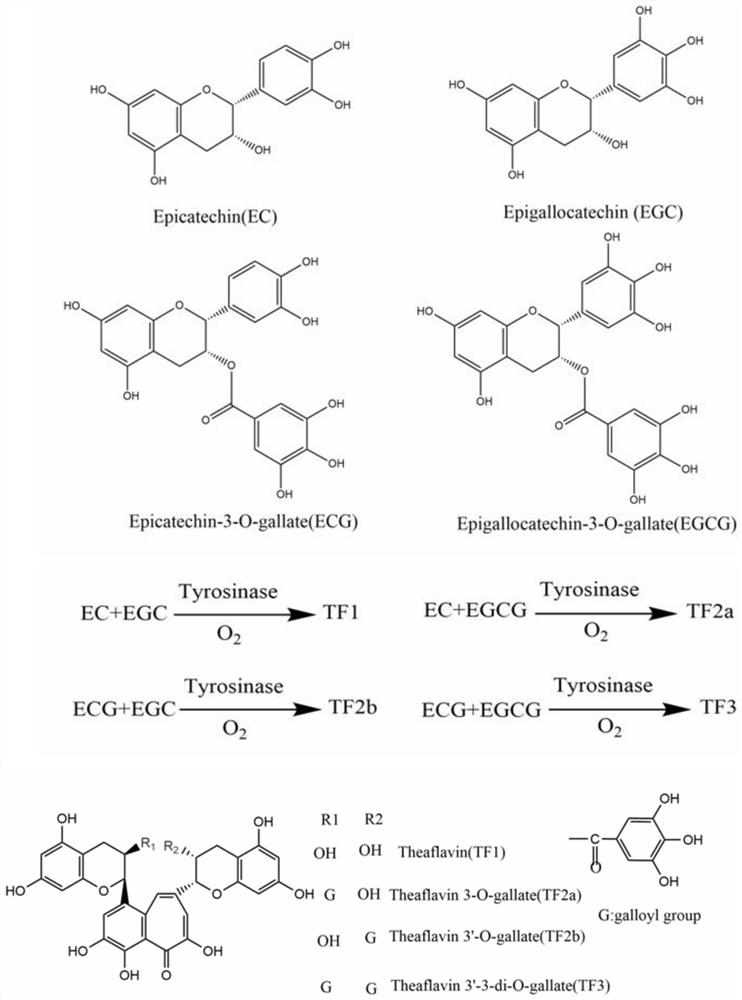
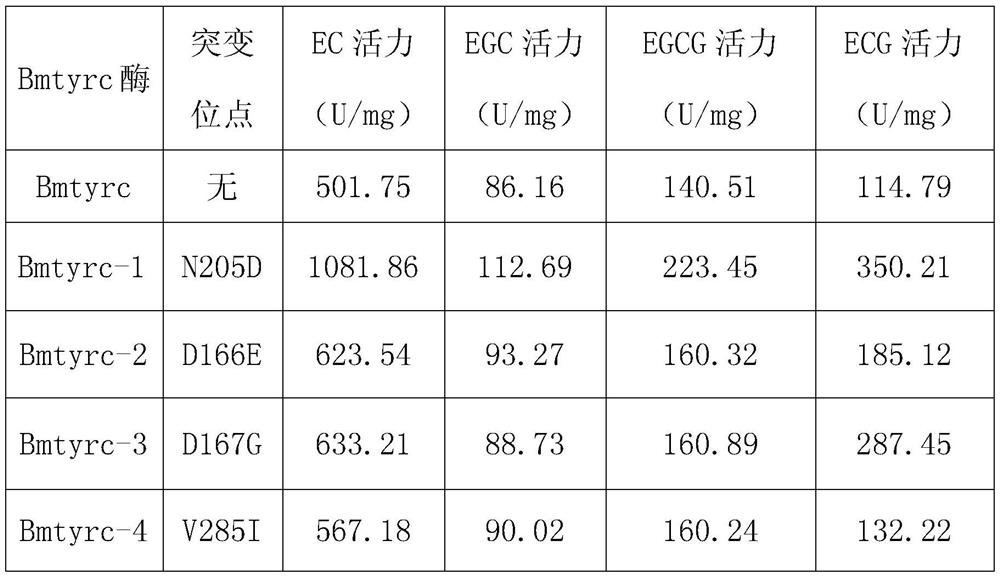
Tyrosinase mutant and application thereof
A kind of tyrosinase and mutant technology, applied in the field of tyrosinase mutants and the synthesis of theaflavins using the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Construction of prokaryotic expression strain of tyrosinase Bmtyrc derived from Bacillus megaterium
[0037] Download the amino acid sequence of tyrosinase derived from Bacillus megaterium in GenBank (SEQ ID NO.1 in this article, corresponding to GenBank accession number: ACC86108.1), and submit the amino acid sequence to Beijing Qingke Biotechnology Co., Ltd. for complete gene sequence synthesis (using large intestine Bacillus preferred codon). The C-terminal of the synthetic gene has a His tag, and it is constructed into the prokaryotic expression vector pET30a(+). The prokaryotic expression vector restriction site: Nde I at the 5' end, Xho I at the 3' end. Pass the constructed plasmid pET30a(+)-Bmtyrc through CaCl 2 Transformed into Escherichia coli expression strain BL21(DE3) by heat shock transformation method, spread on LB solid medium plate containing 50 μg / ml Kanamycin, and cultivate overnight at 37°C, the colony grown on the plate is prokaryotic exp...
Embodiment 2
[0039] Embodiment 2: Purification and immobilization of tyrosinase (Bmtyrc)
[0040] Using the His tag carried in the Bmtyrc recombinant protein, using activated IDA resin (purchased from Anolun (Beijing) Biotechnology Co., Ltd., specific model: His.Bind Resin, Ni-charged), the specific methods and steps used As follows: 4°C, 10000r / min, centrifuge the fermentation broth for 10min, discard the supernatant, collect the bacteria, wash the bacteria twice with phosphate buffer (pH8.0, 0.1mol / L), concentrate the bacteria after centrifugation 5 times resuspended in 20mL phosphate buffer (pH 8.0, 0.1mol / L). The above-mentioned treated bacterial liquid was placed in ice water for ultrasonic crushing until clarification, the ultrasonic crushing conditions were: working for 2s, interval of 5s, ultrasonic power 500W. The crushed lysate was centrifuged in a low-temperature high-speed centrifuge (12000 rpm, 4° C., 20 min), and the supernatant was collected to obtain crude protein. Load t...
Embodiment 3
[0044] Example 3: Construction of Bmtyrc prokaryotic expression strain E.coli BL21(DE3) / pET30a(+)-Bmtyrc error-prone mutation library
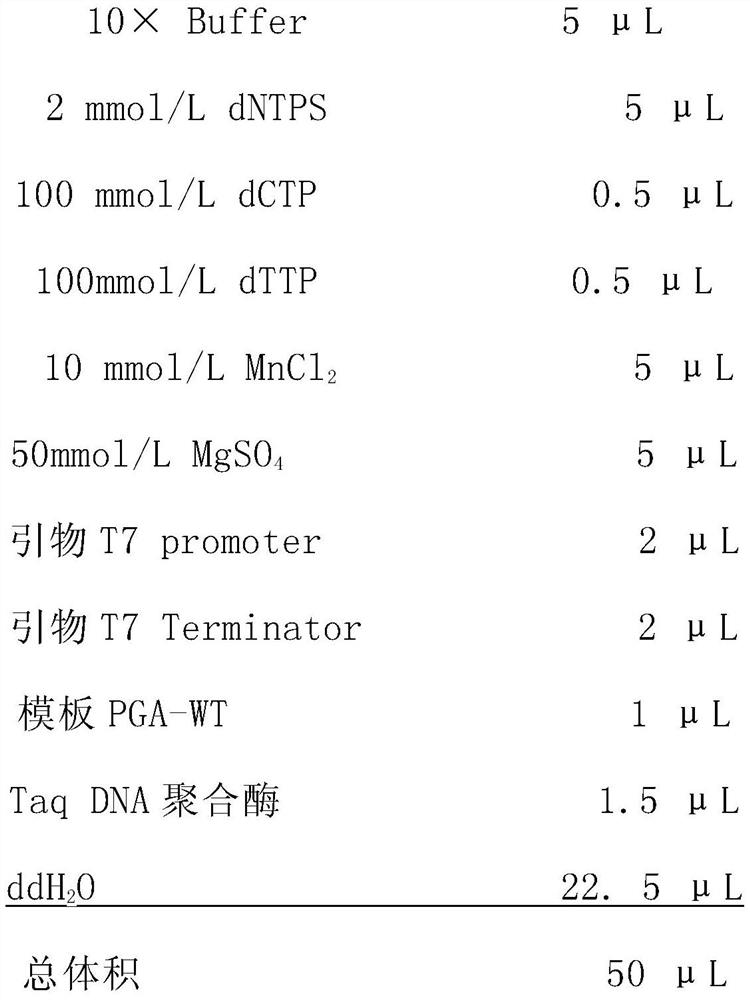
[0045] Using the pET30a(+)-Bmtyrc recombinant plasmid as a PCR template, conventional T7F / R as a universal primer (primer sequence: T7F: 5'-TAATACGACTCACTATAGGG-3', T7R: GCTAGTTATTGCTCAGCGG see SEQ ID NO.12 and 13) for the Bmtyrc gene Perform error-prone PCR amplification and adjust Mg in the PCR amplification reaction system 2+ , Mn 2+ , dCTP and dTTP oligonucleotide concentrations, so that the base mismatch rate of the mutant library is only 2 / 1000, that is, it is guaranteed that only 1 to 2 amino acids are mutated in a mutant.
[0046] Error-prone PCR reaction system:
[0047]
[0048] Error-prone PCR reaction conditions: pre-denaturation at 95°C for 5 minutes; then denaturation at 94°C for 30 seconds, annealing at 56°C for 1 minute, and extension at 72°C for 1.5 minutes, a total of 25 cycles; finally, extension at 72°C for 10 minutes....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com