Pharmaceutical composition for treating cancers, pharmaceutical preparation as well as applications and production methods thereof
A technology of pharmaceutical preparations and compositions, which is applied in the field of pharmaceutical preparations for the treatment of cancer, can solve the problems of large dosage, unclear active ingredients and their mechanism of action, etc., and achieve the effects of fast curative effect, improvement of side effects, and obvious anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation of A1 lyophilization
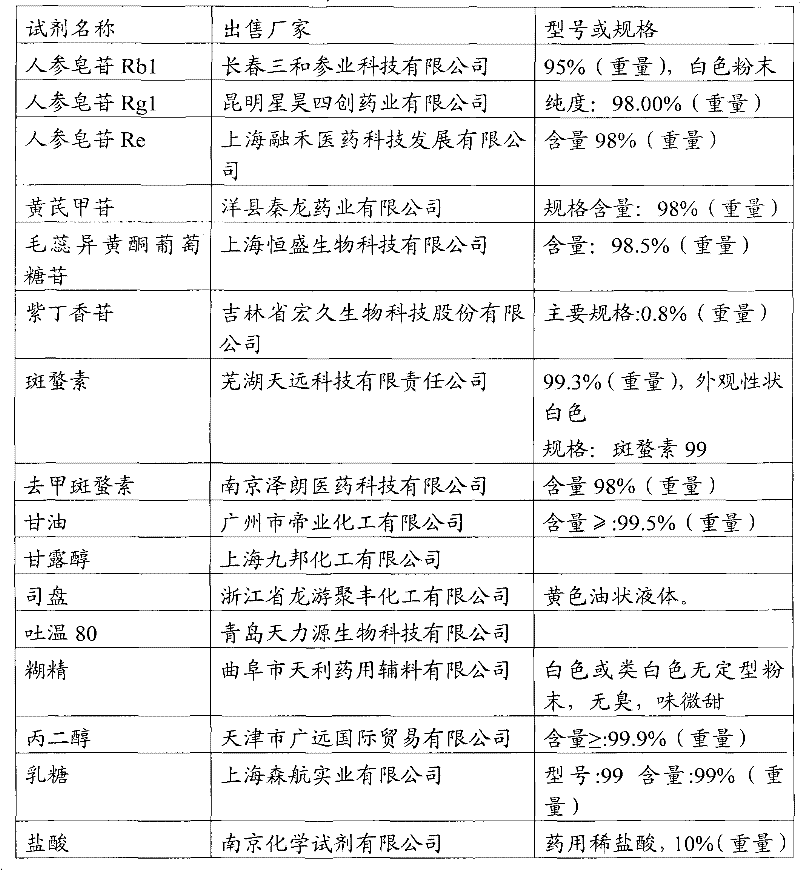
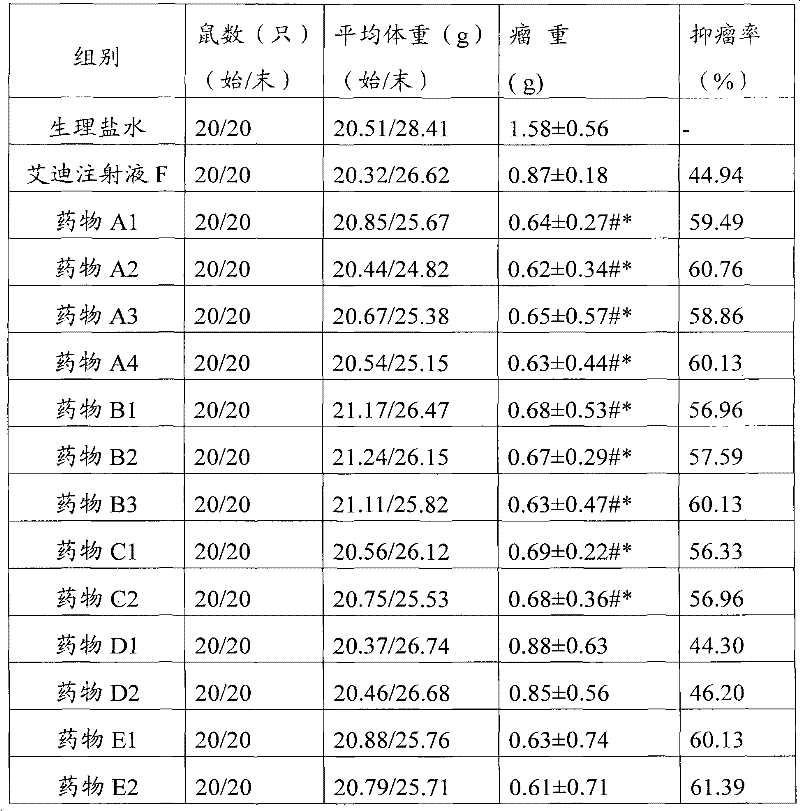
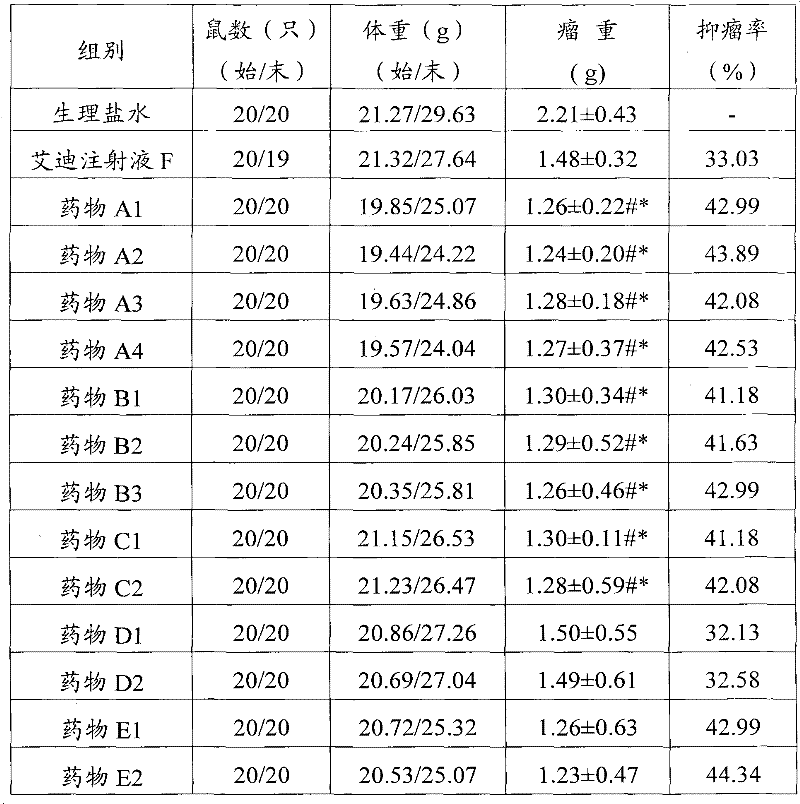
[0055] Take 100 mg of ginsenoside Rb1, 50 mg of ginsenoside Rg1, 100 mg of ginsenoside Re, 40 mg of astragaloside IV, 20 mg of versiflavone glucoside, 75 mg of syringin, and 5.25 mg of cantharidin, and add them to 5 ml of glycerin. After heating and dissolving in a water bath, add 5 ml of Span and mix well, then add 40g mannitol, add water for injection to dissolve and make up to 200ml, adjust the pH value to 3 with dilute hydrochloric acid, filter, fill in 5ml vials, 2ml each, freeze-dried, capped, 100 finished products were prepared, and each active ingredient was about 3.90 mg.
Embodiment 2
[0056] Example 2: Preparation of A2 lyophilization
[0057] Take 100 mg of ginsenoside Rb1, 50 mg of ginsenoside Rg1, 100 mg of ginsenoside Re, 40 mg of astragaloside IV, 20 mg of Verisoflavone glucoside, 75 mg of syringin, 6.3 mg of cantharidin, and 70 mg of norcantharidin, added to 20 ml of propylene glycol, and water bath. After heating and dissolving, add 20ml of Tween 80 and mix well, then add 120g of dextrin, add water for injection to dissolve and dissolve to 200ml, adjust the pH value to 3 with dilute hydrochloric acid, filter, and fill in 5ml vials, 2ml per bottle , freeze-drying, and capping to obtain 100 finished products, each of which is about 4.61 mg of active ingredient.
Embodiment 3
[0058] Example 3: Preparation of lyophilized A3
[0059] Take 90 mg of ginsenoside Rb1, 45 mg of ginsenoside Rg1, 110 mg of ginsenoside Re, 48 mg of astragaloside IV, 18 mg of Verisoflavone glucoside, 67.5 mg of syringin, and 5.8 mg of cantharidin, and add them to 10 ml of glycerin. After heating and dissolving in a water bath, add Mix 10ml of Tween 80, then add 60g of lactose, add water for injection to dissolve and make up to 200ml, adjust the pH to 4 with dilute hydrochloric acid, filter, fill in 5ml vials, 2ml per bottle, freeze-dried, capped , to obtain 100 finished products, each active ingredient is about 3.84mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com