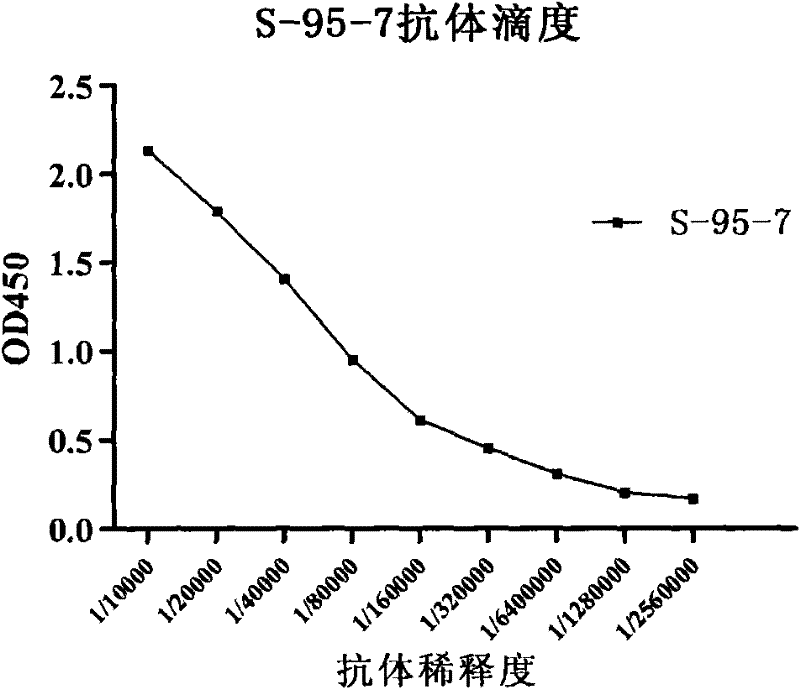
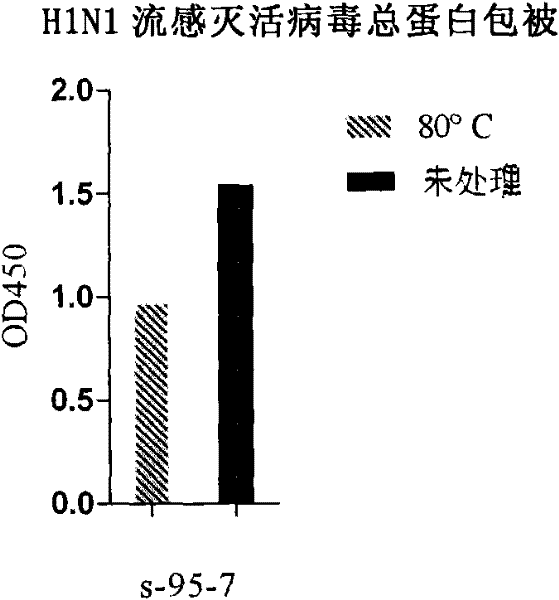
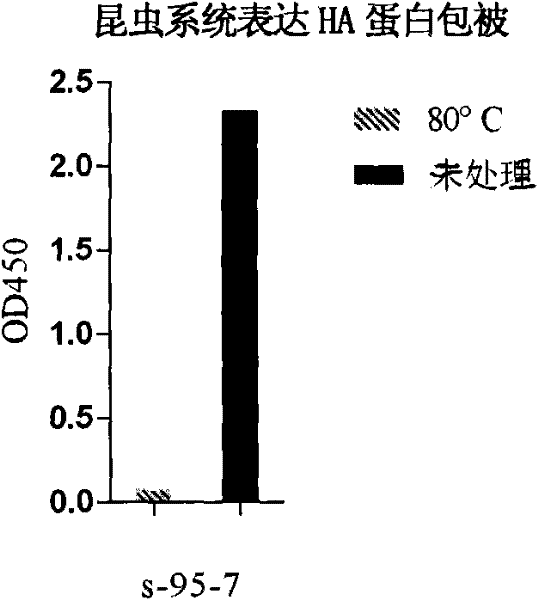
Antibody against hemagglutinin of influenza A H1N1 virus
A technology of hemagglutinin protein and influenza virus, which is applied in the field of antibodies against H1N1 influenza virus hemagglutinin protein, and can solve the problem of no specific monoclonal antibody for H1N1 influenza virus hemagglutinin protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] Preparation of monoclonal antibodies
[0051] The monoclonal antibody of the present invention is prepared using hybridoma technology (see Kohler et al., Nature 256; 495, 1975; Kohler et al., Eur.J.Immunol.6:511, 1976; Kohler et al., Eur.J.Immunol.6 : 292, 1976; Hammerling et al., In Monoclonal Antibodies and T Cell Hybridomas, Elsevier, N.Y., 1981). The preparation method of a kind of hybridoma cell of monoclonal antibody of the present invention is: (1) utilize type A H1N1 influenza virus vaccine to immunize mice; (2) separate the splenocytes of immunized mice, and SP2 / 0 myeloma Cell line fusion; (3) adding mouse peritoneal feeder cells to co-culture with fusion cells, and screening positive strains; (4) cloning and screening by limiting dilution method to obtain monoclonal antibody cell lines, and screening from the cell lines can A cell line producing the desired monoclonal antibody.
[0052] The monoclonal antibody of the present invention can also be obtained by...
Embodiment approach
[0053] After the hybridoma cells are obtained, monoclonal antibody production techniques well known to those skilled in the art can be used to mass-produce the monoclonal antibody of the present invention. As an embodiment of the present invention, the monoclonal antibody can be prepared by the following preparation method, which includes the steps of: (1) providing adjuvant pretreated mice; (2) intraperitoneally inoculating the mice The hybridoma cells secrete monoclonal antibodies; (3) ascitic fluid is extracted, and the monoclonal antibodies are obtained by separation. As a way, the method of isolating monoclonal antibody from ascites is: collect ascites, precipitate with ammonium sulfate, and then use Protein G prepacked chromatographic column to purify to obtain high-purity anti-H1N1 influenza virus HA monoclonal antibody .
[0054] In addition, the hybridoma cells can also be cultured and expanded in vitro according to conventional animal cell culture methods, so as to ...
Embodiment 1
[0088] Example 1. Preparation of anti-H1N1 influenza virus hemagglutinin protein (HA) monoclonal antibody
[0089] 1. Influenza A (H1N1) virus vaccine
[0090] In the present invention, influenza A H1N1 virus vaccine (obtained from Hualan Biotechnology Co., Ltd., concentration 100ug / ml) was used to immunize BALB / c mice to prepare monoclonal antibodies.
[0091] 2. Animal immunization
[0092] Take 100 μg of H1N1 influenza virus vaccine mixed with an equal volume of Freund's adjuvant, emulsify completely, and inject subcutaneously on the back and feet of BALB / c mice to immunize mice. Vaccine once every 3 weeks, and 3 times in a row. Complete Freund's adjuvant was used for the first immunization, and incomplete Freund's adjuvant was used for the second and third immunizations. One week after the third immunization, blood was collected from the orbit, the serum was separated, and the titer of anti-H1N1 influenza virus vaccine antibody was measured. A mouse with a high titer w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com