Method for synthesizing 3,5-diamido benzoic acid by industrial continuous hydrogenation
A technology of diaminobenzoic acid and dinitrobenzoic acid, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of not disclosing substantive content, affecting the number of times of application, and being unable to apply. Significant economic and social benefits, reducing the use of metals, and ensuring product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
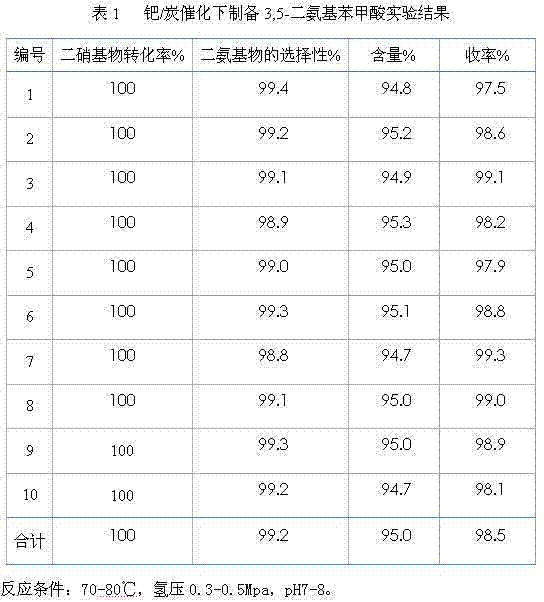
[0032] Take 100 kilograms of m-dinitrobenzoic acid to produce 3,5-diaminobenzoic acid as example, the catalyst used is the palladium / carbon of loading 3wt%, and the total amount of catalyst used in the production process is 3% (3 kilograms) of raw material, The total amount of water is 8.5 times (850 kg) of the raw material. During continuous production, dinitrobenzoic acid, water and catalyst are added according to the flow rate. The specific process is as follows:
[0033] 1. Start the feeding pump, adjust the flow rate to 540 L / h, and pass it into the primary reduction kettle at this flow rate, and at the same time, pass the catalyst (3wt% of the raw material) and water (8.5 times the mass of the raw material) into the primary reduction kettle In the process, adjust the stirring speed of the first-stage tank to about 200 rpm, and then introduce hydrogen to carry out the reduction reaction. The hydrogen reaction starts at 0.1Mpa. In this embodiment, the hydrogen pressure is k...
Embodiment 2
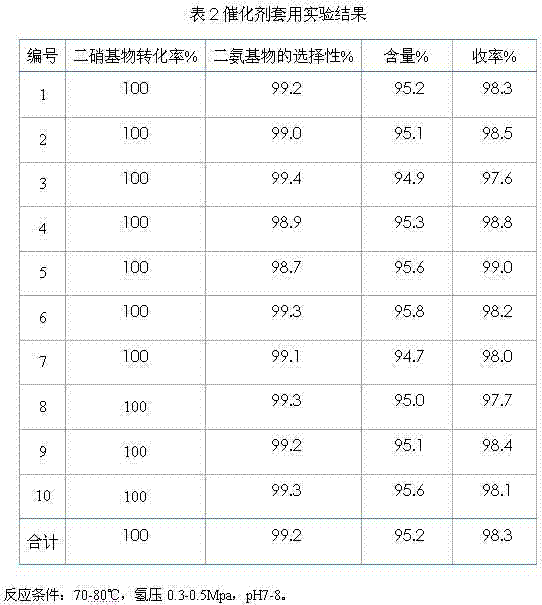
[0040] Production of 3,5-diaminobenzoic acid with 100 kilograms of m-dinitrobenzoic acid is an example, the catalyst used in the production process is the palladium / carbon of loading 3wt%, and consumption is 3% (3 kilograms) of m-dinitrobenzoic acid ), the total amount of water is 8.5 times (850 kilograms) of raw material, and during continuous production, dinitrobenzoic acid, water and catalyzer are added according to flow velocity flow, react according to the continuous production process of embodiment 1, in order to investigate The stability of technology, carry out ten experiments continuously according to above-mentioned method, and its result is as follows:
[0041]
[0042] As can be seen from the above table, the conversion rate of 3,5-diaminobenzoic acid reaches 100%, the selectivity of diamino is more than 98%, and the average can reach more than 99%, and the average content of the final product GPLC external standard method can be It can reach 95% (including 5% s...
Embodiment 3
[0044] Taking 100 kilograms of m-dinitrobenzoic acid to produce 3,5-diaminobenzoic acid as an example, the catalyst used in the production process is palladium / carbon with a load of 3wt%, and the dosage is 3% (3 kg) of m-dinitrobenzoic acid , the total amount of water is 8.5 times (850 kilograms) of raw material, and during continuous production, dinitrobenzoic acid, water and catalyst are added according to flow rate flow, react according to the continuous production process of embodiment 1, difference is The catalyst is recycled, which is embodied in step 4: the reaction liquid flowing out from the three-stage tank overflows into the settler, and the reducing liquid entrained with the catalyst is separated from the solid and liquid after settling. When the liquid level of the settler reaches 83% , open the discharge valve of the settler, open the self-regulating valve, the liquid layer with a low solid content in the settler enters the filter, and the reducing solution that f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com