Preparation method of difenoconazole
A technology of difenoconazole and chlorophenyl ether, which is applied in the field of preparation of difenoconazole, can solve the problems of low conversion rate of bromination reaction, difficult to increase condensation temperature, low bromide content, etc., and achieve shortened condensation Reaction time, increased condensation reaction temperature, and high yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
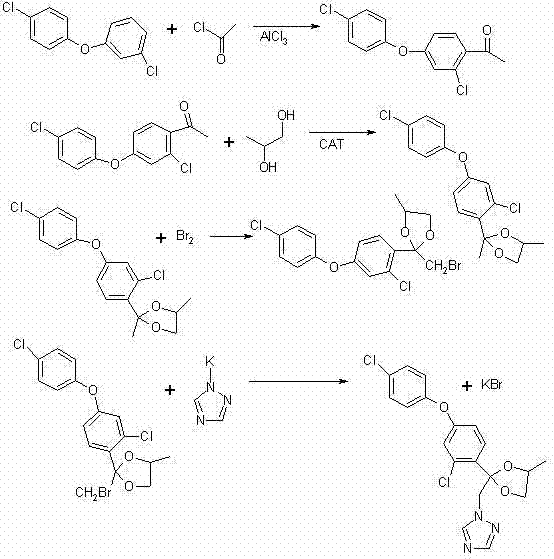
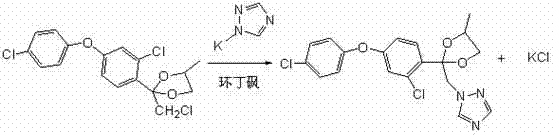
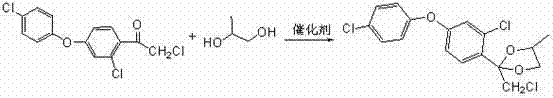
[0024] The preparation method of difenoconazole according to the present embodiment comprises the following four steps carried out in sequence:
[0025] (1), Friedel-Crafts reaction
[0026] ① Reaction equation:
[0027]
[0028] ② Specific implementation process: Add 500 ml of dry dichloroethane into a 1000 ml three-necked flask, and at the same time add 134 grams of anhydrous aluminum trichloride and stir for 30 minutes, then add 103 grams of chloroacetyl chloride, and keep stirring at 20-25 ° C After 30 minutes, cool in an ice bath to 0-5°C and add 183 grams of 3,4'-dichlorodiphenyl ether dropwise. The reaction is exothermic during the dropwise addition, and the dropping rate is controlled to keep the reaction temperature between 5°C and 10°C. After about 2 hours, the dropwise addition is completed, then remove the ice bath and slowly heat to 25°C-30°C for 2 hours, then take a sample for analysis, when the content of 3,4'-dichlorodiphenyl ether is less than 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com