Method for preparing nonmetal-doped TiO2 photocatalyst
A photocatalyst, non-metallic technology, applied in the field of preparation of TiO2 photocatalyst, can solve the problems of increasing the amount of combustion agent and auxiliary agent, difficult to control, uncontrollable doping ratio, etc., and achieves the improvement of photocatalytic activity and response performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
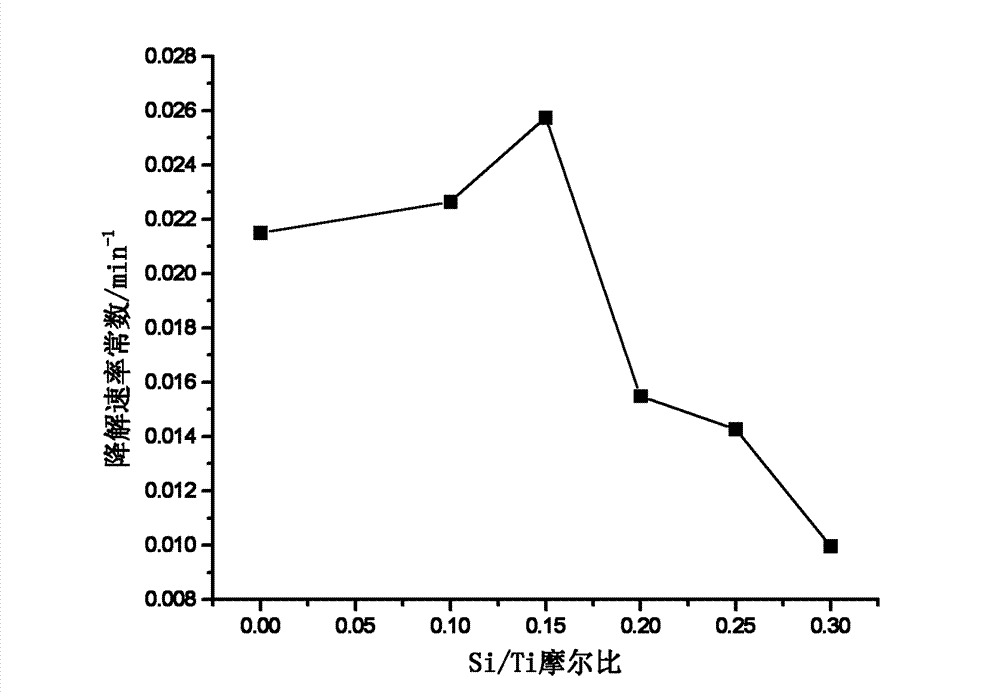
[0027] Si-doped TiO 2 Preparation of photocatalyst: Prepare 6 parts of the same sol according to the ratio of butyl titanate: water: acetic acid: n-butanol = 1:3:5:8, and then according to the Si / Ti molar ratio of 0, 0.10, 0.15, Add silicon powder at 0.20, 0.25, and 0.30, stir well, and evaporate to gel in vacuum. Put the gel into the muffle furnace, continuously feed sufficient oxygen, and raise the temperature at a rate of 1-5°C until the gel spontaneously ignites, and the spontaneous combustion process can maintain the heat required for the system to heat up. After full combustion, non-metallic Si-doped TiO can be obtained 2 . Continue heat treatment at 500°C for 2 hours to obtain Si-doped TiO 2 catalyst of light. Si-doped TiO with various Si / Ti molar ratios 2 Photocatalyst, under the irradiation of low-pressure mercury lamp, the rate of degradation of methylene blue solution changes as figure 1 As shown, it can be seen from the figure that the optimum Si / Ti molar rat...
Embodiment 2
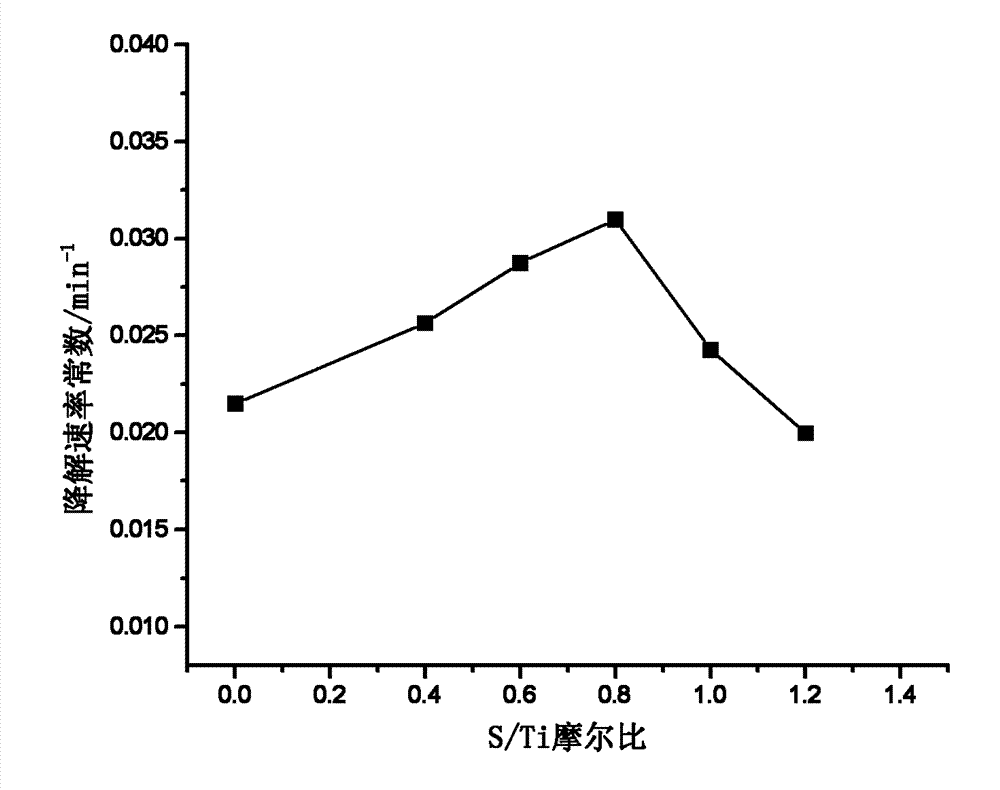
[0029] According to the method of Example 1, adding sulfur powder according to the S / Ti molar ratio of 0, 0.40, 0.60, 0.80, 1.00, 1.20, S-doped TiO can be obtained 2 catalyst of light. S-doped TiO with various S / Ti molar ratios 2 Photocatalyst, under the irradiation of low-pressure mercury lamp, the rate of degradation of methylene blue solution changes as figure 2 As shown, it can be seen from the figure that the optimal S / Ti molar ratio is 1.00, and the S-doped TiO 2 The rate constant of catalyst degradation methylene blue is 0.031min -1 , is pure TiO 2 1.43 times.
Embodiment 3
[0031] According to the method of Example 1, according to the optimal doping ratio obtained respectively in Examples 2 and 3, silicon powder and sulfur powder are simultaneously doped in the sol according to the Si / Ti molar ratio of 0.15 and the S / Ti molar ratio of 1.00 , can make Si / S co-doped TiO2. The rate constant of the catalyst for the degradation of methylene blue is 0.035min -1 , is pure TiO 2 1.61 times.
[0032] According to the description of the above examples, changing the combustion agent can prepare N, P, S, Si, C and other non-metal doped TiO 2 catalyst of light.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com