A kind of preparation method of difluorooxalate lithium borate electrolyte salt
A technology of lithium difluorooxalate borate and electrolyte salt, applied in secondary batteries, chemical instruments and methods, circuits, etc., can solve the problems of large SEI film resistance, high investment cost, staff health and environmental hazards, etc. Simple process, low corrosiveness, environment-friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
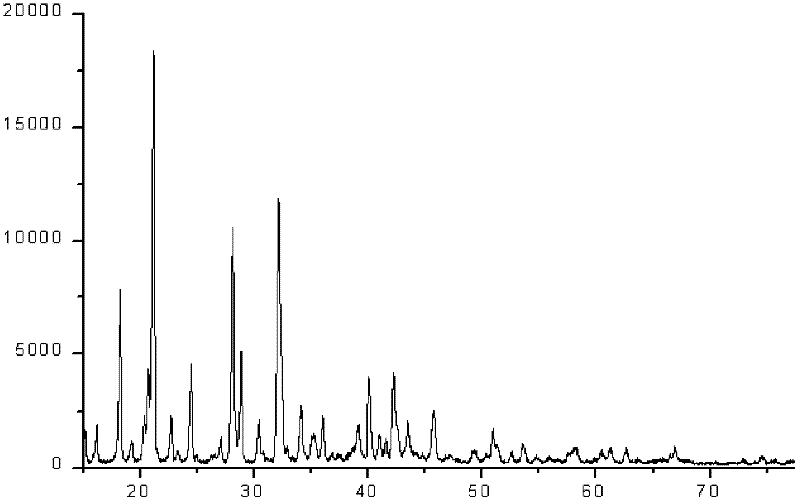
Image
Examples
Embodiment 1
[0023] Lithium difluorooxalate borate was prepared by using lithium fluoride, boric acid and oxalic acid as raw materials.
[0024] Step 1: Put 30.9 g of boric acid and 26 g of lithium fluoride into a ball mill jar, mix and ball mill for 12 hours at 5° C., and the speed of the planetary ball mill is 380 rpm.
[0025] Step 2: Put the ball-milled raw materials above in a muffle furnace, raise the temperature to 350° C. under the protection of nitrogen, and keep the temperature constant for 20 hours to make them react completely.
[0026] Step 3. Transfer the above-mentioned treated raw materials to an appropriate amount of water, stir while heating, and then add 94.8 g of oxalic acid after the mixture is evenly mixed. After the reaction is complete, put the solution into a blast drying oven to remove water to obtain a white product.
[0027] Step 4. Grind the white product finely, then put it into a container, add 1500g of dimethyl carbonate, heat and stir after sealing the cont...
Embodiment 2
[0029] Lithium difluorooxalate borate was prepared by using lithium fluoride, diboron trioxide and oxalic acid as raw materials.
[0030] Step 1: Put 17.5 g of diboron trioxide and 26 g of lithium fluoride into a ball mill jar, mix and ball mill for 12 hours at 5° C., and the speed of the planetary ball mill is 380 rpm.
[0031] Step 2: Put the ball-milled raw materials above in a muffle furnace, raise the temperature to 400° C. under the protection of nitrogen, and keep the temperature constant for 20 hours to make them react completely.
[0032] Step 3. Transfer the above-mentioned processed raw materials to an appropriate amount of water, stir while heating, and then add 94.8 g of oxalic acid after the mixture is evenly mixed. After the reaction is complete, put the solution into a blast drying oven to remove water to obtain a white product.
[0033] Step 4: Grind the white product finely, then put it into an open reactor, add 1500g of dimethyl carbonate, heat and stir afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com