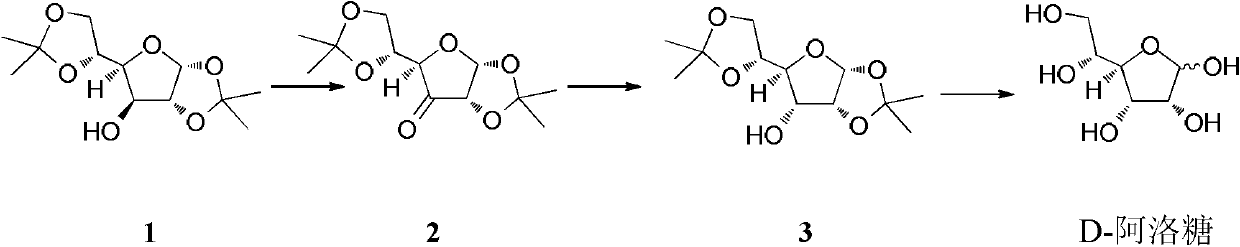
A method for preparing d-allose by reducing ketose by catalytic hydrogenation
A kind of technology of allose and catalyst, applied in the field of preparing D-allose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, preparation D-allose
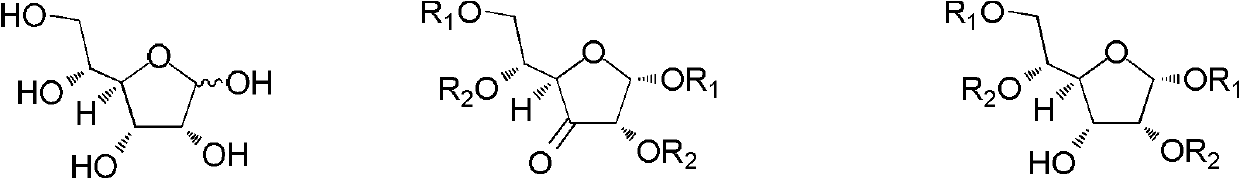
[0035] A mixed system containing 10g Raney Ni (Raney nickel), 200mL methanol and 10g 1,2:5,6-O-dipropylidene-3-deoxy-3-oxo-α-D-glucose 2 was dissolved in 30atm hydrogen React under pressure for 72 hours at a reaction temperature of 28-35°C. The catalyst was filtered off, and the filtrate was spin-dried to obtain a pale yellow oily liquid. The product has been 1 H NMR detection found that it contained 1,2:5,6-O-dipropylidene-α-D-glucose 1 (content 18%) and 1,2:5,6-O-dipropylidene-α-D-A Lulose 3 (content 82%). The conversion efficiency was 82%. The "transformation efficiency" in the present invention refers to the 1,2:5,6-O-dipropylidene-α-D-allose 3 produced relative to the 1,2:5,6-O- Ratio of bispropylidene-3-deoxy-3-oxo-α-D-glucose 2.
[0036] The NMR characterization of 1,2:5,6-O-dipropylidene-α-D-allose 3 is as follows: 1 H-NMR (300MHz, CDCl 3 ), δ (ppm), 5.82 (1H, d, J = 3.8Hz), 4.62 (1H, dd, J = 5.0Hz, 4.1Hz), 4.30 (1H, ...
Embodiment 2
[0038] Embodiment 2, preparation D-allose
[0039] A mixed system containing 10mg Pd / C (10%), 20mL ethanol and 100mg 1,2:5,6-O-dipropylidene-3-deoxy-3-oxo-α-D-glucose 2 was dissolved in 50atm hydrogen React under pressure for 72 hours, and the reaction temperature is 28-35°C. The catalyst was filtered off, and the filtrate was spin-dried to obtain a pale yellow oily liquid. The product has been 1 H NMR detection revealed that it contained unreacted ketose 2 (content 86%) and 1,2:5,6-O-dipropylidene-α-D-allose 3 (content 14%). The conversion efficiency was 100%. The product was recrystallized in a mixed solvent of ethyl acetate / petroleum ether, and then separated by column chromatography (mobile phase: ethyl acetate / petroleum ether=1 / 3) to obtain 1,2:5,6-O-bispropylidene -α-D-Allose 3.
[0040] The resulting reduction product 1,2: 5,6-O-dipropylidene-α-D-allose 3 was reacted at 90°C for 12 hours in the presence of an acidic resin in the presence of a macroporous strongly a...
Embodiment 3
[0041] Embodiment 3, preparation D-allose
[0042] 10mg contains Pd(OH) 2 , 10mL of methanol and 20mg of 1,2:5,6-O-dipropylidene-3-deoxy-3-oxo-α-D-glucose 2 was reacted under 50atm hydrogen pressure for 48 hours, and the reaction temperature was 30- 40°C. The catalyst was filtered off, and the filtrate was spin-dried to obtain a pale yellow oily liquid. The product has been 1 H NMR detection found that it contained 1,2:5,6-O-dipropylidene-α-D-glucose 1 (content 6%), unreacted ketose 2 (content 66%) and 1,2:5,6 -O-dipropylidene-α-D-allose 3 (content 28%). The conversion efficiency was 81%. The product was recrystallized in ethyl acetate / petroleum ether mixed solvent, and then separated by column chromatography (mobile phase: ethyl acetate / petroleum ether=1 / 3, v / v) to obtain 1,2:5,6-O - Dipropylidene-α-D-allose 3.
[0043] The resulting reduction product 1,2: 5,6-O-dipropylidene-α-D-allose 3 was reacted at 90°C for 12 hours in the presence of an acidic resin in the presen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com