an interventional medical device
一种医疗器械、涂层的技术,应用在医疗器械领域,能够解决生物相容性差、血栓及术后并发症、复杂生物相容性等问题,达到细胞相容性增强的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
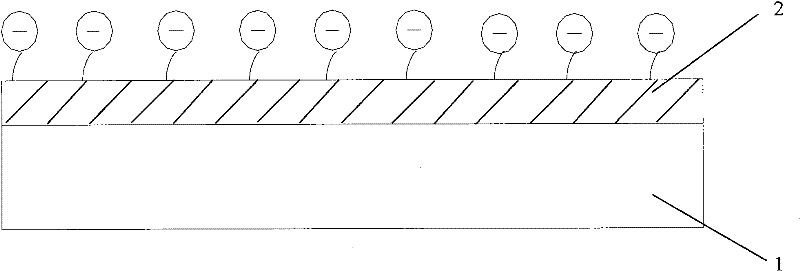
[0035] figure 1 It is a schematic structural diagram of an interventional medical device provided in Embodiment 1 of the present application.
[0036] Such as figure 1 As shown, the interventional medical device includes a stent body 1 and a coating 2 disposed on the outer surface of the stent body 1, wherein: the material of the coating 2 is a polymer containing sulfonic acid groups.
[0037] In the embodiment of the present application, the polymers containing sulfonic acid groups used in coating 2 include but are not limited to sulfonated thermoplastic elastomers, preferably sulfonated styrene-olefin copolymers, more preferably styrene-isobutylene di Block or tri-block copolymer, wherein the weight percentage of styrene is 25-55%. In addition, the sulfonated thermoplastic elastomer can also be other copolymers well known to those skilled in the art.
[0038] In the embodiment of the present application, the sulfonation rate in the polymer containing sulfonic acid groups ...
Embodiment 2
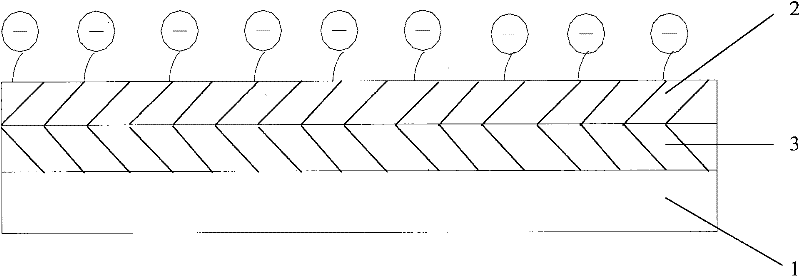
[0041] figure 2 It is a schematic structural diagram of an interventional medical device provided in Embodiment 2 of the present application.
[0042] Such as figure 2 As shown, the interventional medical device includes a stent body 1, an outermost coating 2 and an inner coating 3 coated on the outer surface of the stent body 1, wherein: the material of the outermost coating 2 is a material containing a sulfonic acid group polymer, and the sulfonation rate of the polymer containing sulfonic acid groups used in the outermost coating layer 2 is 5% to 30%, more preferably 10% to 20%.
[0043]The material of inner layer coating 3 can be inorganic coating, organic coating or traditional polymer coating, also can be the polymer coating that contains sulfonic acid group, when the material of inner layer coating 3 is to contain sulfonic acid group When using agglomerated polymers, since the environment of the inner coating 3 and the outermost coating 2 are different, the requirem...
Embodiment 3
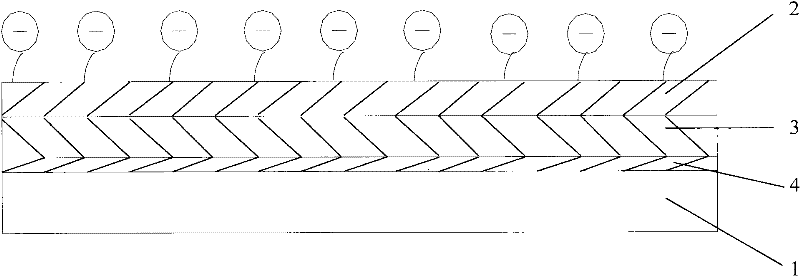
[0045] image 3 It is a schematic structural diagram of an interventional medical device provided in Example 3 of the present application.
[0046] Such as image 3 As shown, on the basis of the second embodiment, a layer of primer coating 4 may be added between the inner coating layer 3 and the stent body 1 as required.
[0047] Like the inner coating 3 in the second embodiment, the material of the bottom coating 4 can also be an inorganic coating, an organic coating or a traditional polymer coating, and can also be a polymer coating containing sulfonic acid groups. When the material of the primer coating 4 is a polymer containing sulfonic acid groups, the sulfonation rate of the polymer containing sulfonic acid groups in the primer coating 4 should be controlled below 15%, preferably 5-10%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com