Porous iron oxide structured by nanocrystals and preparation method thereof
A technology of nano-iron oxide and iron oxide, which is applied in the direction of iron oxide, iron oxide/iron hydroxide, etc., can solve the problems of soft template method residue and hard template method preparation method, and achieve the effect of simple and controllable method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
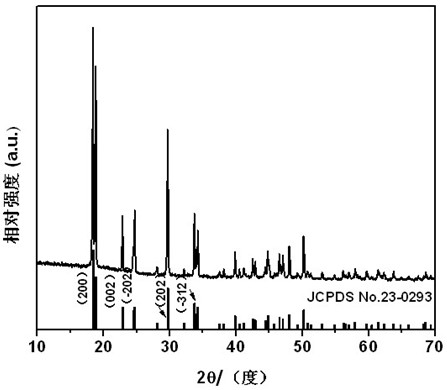
[0020] Dissolve 1.47g of potassium oxalate in 40.0mL of deionized water and stir to make it evenly dispersed in the solution. The concentration of oxalate is 0.2mol / L; dissolve 3.14g of ammonium ferrous sulfate in 40.0mL of deionized water and stir to make it evenly Dispersed in the solution, the concentration of ferrous ammonium sulfate is 0.2mol / L. Then slowly add the potassium oxalate solution into the ferrous ammonium sulfate solution under constant stirring, the ratio of ferrous ions to oxalate ions is 1:1. After the reaction was completed, it was transferred to a 100 mL hydrothermal kettle, and reacted at 120° C. for 10 hours, and the obtained product was dried at 80° C. for 5 hours to obtain a strip-shaped precursor. figure 1 It is the XRD pattern of the precursor, which is consistent with the standard ferrous oxalate XRD data (JCPDS No. 23-0293), indicating that the crystalline phase of the precursor is ferrous oxalate. The dried precursor ferrous oxalate was placed i...
Embodiment 2
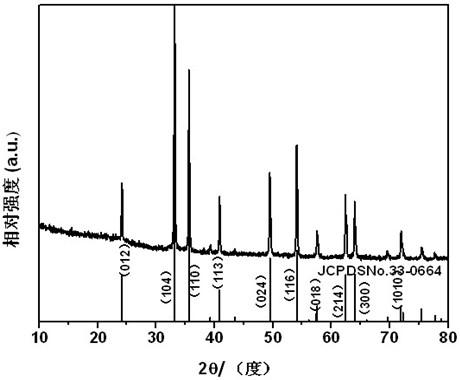
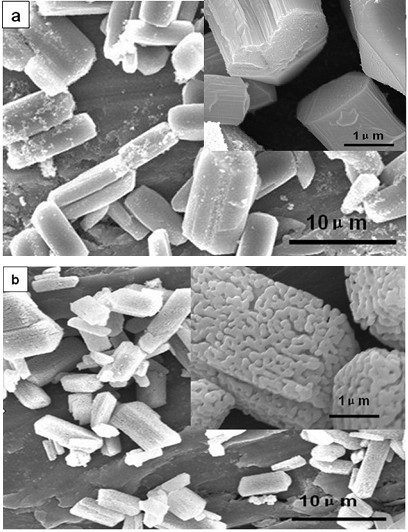
[0022] Dissolve 0.88g potassium oxalate in 40.0mL deionized water, stir to make it evenly dispersed in the solution, the concentration of oxalate is 0.12mol / L; dissolve 1.12g ferrous sulfate in 40.0mL deionized water, stir to make it evenly Dispersed in the solution, the concentration of ferrous sulfate is 0.1mol / L. Then slowly add the potassium oxalate solution into the ferrous sulfate solution under constant stirring, and the ratio of ferrous ions to oxalate ions is 1.2:1. After the reaction was completed, it was transferred to a 100 mL hydrothermal kettle and reacted at 100° C. for 12 hours, and the obtained product was dried at 80° C. for 5 hours to obtain a strip-shaped precursor. The dried precursor ferrous oxalate was placed in a muffle furnace, the temperature was raised to 600°C at a heating rate of 5°C / min and kept for 4 hours, and then slowly cooled to room temperature with the furnace temperature to obtain nanostructured porous iron oxide. The electron microscope ...
Embodiment 3
[0024] Dissolve 0.37g sodium oxalate in 40.0mL deionized water, stir to make it evenly dispersed in the solution, the concentration of oxalate is 0.05mol / L; dissolve 0.800g ammonium ferrous sulfate in 40.0mL deionized water, stir to make it Evenly dispersed in the solution, the concentration of ferrous ammonium sulfate is 0.05mol / L; then slowly add the sodium oxalate solution into the ferrous ammonium sulfate solution under continuous stirring, the content of ferrous ions and oxalate ions The volume ratio is 1:1. After the reaction was completed, it was transferred to a 100 mL hydrothermal kettle, and reacted at 80° C. for 8 hours, and the obtained product was dried at 80° C. for 5 hours to obtain a strip-shaped precursor. The dried precursor ferrous oxalate was placed in a muffle furnace, and the temperature was raised to 600°C at a heating rate of 2°C / min and kept for 4 hours, and then slowly cooled to room temperature with the furnace temperature to obtain nanostructured po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com