Thiadiazole compound as well as preparation and application thereof
A technology of thiadiazoles and compounds, applied in the field of thiadiazoles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the synthesis of target compound
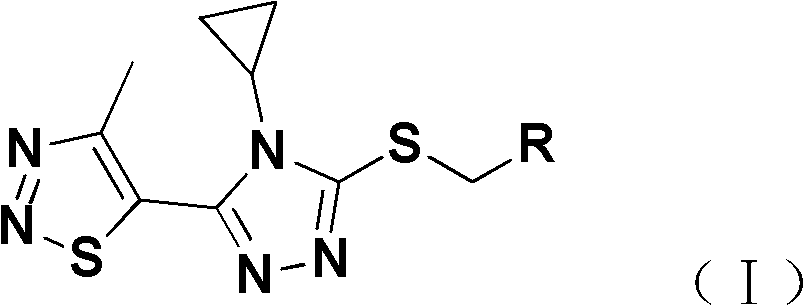
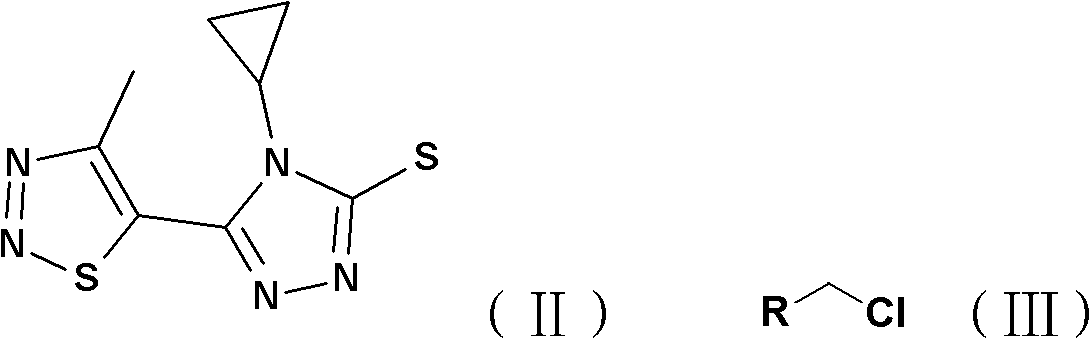
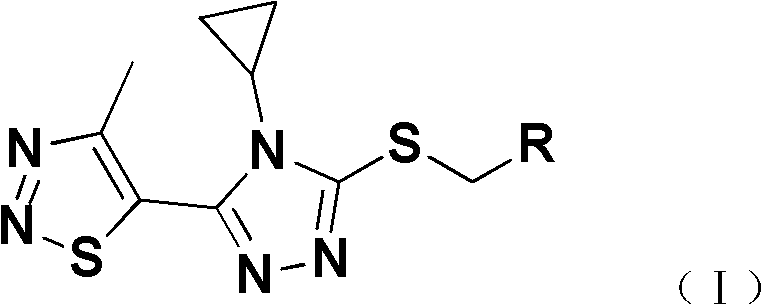
[0024] Add 2mmol 4-cyclopropyl-5-(4-methyl-1,2,3-thiadiazol-5-yl)-4H-1,2,4-triazole-3-sulfur to a 25mL round bottom flask Alcohol (II), 2mmol substituted benzyl chloride (III), 8mL DMF and 2.4mmol of K 2 CO 3 , stirred at room temperature, and TLC was used to detect the progress of the reaction. After the reaction was completed, the reaction solution was poured into 30 mL of ice water, and the solid was precipitated, filtered, precipitated, and subjected to silica gel column chromatography to obtain a solid product (see Table 1 and Table 2 for the physical and chemical parameters and mass spectrometry data of the target compound).
[0025]
[0026] Table 1: Physicochemical parameters of target compounds
[0027]
[0028]
[0029] Table 2: Target compounds 1 H NMR data
[0030]
[0031]
Embodiment 2
[0032] Example 2: Herbicidal Activity Test
[0033] (1) Rapeseed plate method:
[0034] Spread a piece of filter paper with a diameter of 5.6 cm in a petri dish with a diameter of 6 cm, add 2 ml of a certain concentration of the test compound solution, and sow 10 rapeseed seeds soaked for 4 hours. The radicle length was measured after 72 hours of dark cultivation at 28±1°C. The herbicidal activity of the compounds was detected by the growth inhibition of the radicles of rapeseed under dark conditions. Test concentration: 10 μg / mL and 100 μg / mL. Each treatment was repeated twice. Activity index: radicle elongation inhibition rate (%). Activity grading indicators: Grade A: ≥80%; Grade B: 60-79%; Grade C: 40-59%; Grade D: ≤39%.
[0035] (2) Barnyard grass small cup method:
[0036] After laying glass beads and filter paper in the small beaker of 50 milliliters, add 6 milliliters of the test compound solution of certain concentration, sow 10 barnyardgrass seeds that just dew...
Embodiment 3
[0041] Embodiment 3: Bactericidal activity test
[0042] 1. Test conditions
[0043] 1.1 Crops and targets
[0044] Test crop: cucumber (Cucumis sativus L.), variety: Xintaimici.
[0045] Test objects: cucumber scab (Cladosporium cucumerinum) spore suspension, cucumber brown spot (Corynespora cassiicola) spore suspension, cucumber sclerotinia (Sclerotinia sclerotiorum (Lib.) de Bary) toxic medium method, cucumber powdery mildew ( Erysiphe cichoracearum) spore suspension, cucumber anthracnose (Colletotrichum orbitale (Berk a L Mont) Arx.).
[0046] 1.2 Environmental conditions
[0047] Cucumber seedlings are at the stage of 2 cotyledons.
[0048] 2. Experimental Design and Arrangement
[0049] 2.1 Test drug
[0050] 2.1.1 Test agents and treatment doses
[0051] The entrusting unit provides 139 test samples, and each sample has a test concentration of 500mg / L.
[0052] 2.1.2 Control drug
[0053] The concentration of Zhongshengmycin control agent was also set at 500mg / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com