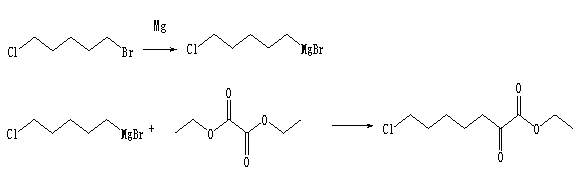
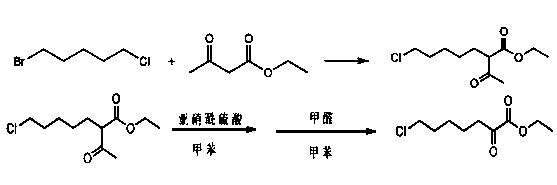
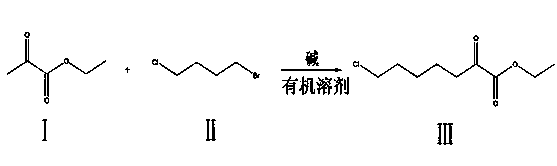
Method for synthesizing 7-chloro-2-oxoheptanoate
A technology of ethyl oxoheptanoate and synthetic method, applied in the synthesis of cilastatin intermediate, the synthetic field of cilastatin intermediate ---7-chloro-2-oxoheptanoate ethyl, can Solve the problems of high cost, harsh reaction conditions, and environmental pollution, and achieve the effects of simple steps, low cost, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Dissolve ethyl pyruvate in tetrahydrofuran, and the mass ratio of ethyl pyruvate to tetrahydrofuran is 1:2 to obtain mixed solvent 1; control the temperature at 60°C, add lithium diisopropylamide dropwise to mixed solvent 1 (in the form of tetrahydrofuran is the solvent), and the time for adding lithium diisopropylamide is controlled to be 0.5 hours to obtain mixed solvent 2; keep the temperature at 60°C, then add 1-bromo-4-chlorobutane to mixed solvent 2, add 1-bromo The time control of -4-chlorobutane is 0.5 hour; Wherein, in molar ratio, the consumption of added ethyl pyruvate, 1-bromo-4-chlorobutane and lithium diisopropylamide is: ethyl pyruvate : 1-bromo-4-chlorobutane: Lithium diisopropylamide=0.5:0.5:2. After adding 1-bromo-4-chlorobutane, keep the temperature of the mixed solvent at 60°C and react for 6 hours; the reaction is completed , filter the reaction product, distill the filtrate under reduced pressure (remove the solvent), and purify by rectification to...
Embodiment 2
[0023] Dissolve ethyl pyruvate in a mixed organic solvent composed of tetrahydrofuran and n-hexane in any proportion. The mass ratio of ethyl pyruvate to the mixed organic solvent is 1:15 to obtain mixed solvent 1; control the temperature at -78°C, Add butyllithium (tetrahydrofuran / n-hexane as solvent) dropwise to mixed solvent 1, and control the time of adding butyllithium to 3 hours to obtain mixed solvent 2; keep the temperature at -78°C, add 1-bromo-4-chlorobutane, the time of adding 1-bromo-4-chlorobutane is controlled as 3 hours; wherein, in molar ratio, the added ethyl pyruvate, 1-bromo-4-chlorobutane The amount of butyllithium and butyllithium is controlled as follows: ethyl pyruvate: 1-bromo-4-chlorobutane: butyllithium=0.5:1:2. After adding 1-bromo-4-chlorobutane, keep the volume of the mixed solvent The temperature was -78°C, and the reaction was carried out for 15 hours; after the reaction was completed, the reaction product was filtered, and the filtrate was disti...
Embodiment 3
[0027] Dissolve ethyl pyruvate in dimethyl sulfoxide, and the mass ratio of ethyl pyruvate to dimethyl sulfoxide is 1:5 to obtain mixed solvent 1; control the temperature at -4°C, and add sodium hydride to the mixed solvent , the time of adding sodium hydride was controlled for 2 hours to obtain mixed solvent 2; keep the temperature at -4°C, add 1-bromo-4-chlorobutane to mixed solvent 2, add 1-bromo-4-chlorobutane The time control is 2 hours; Wherein, by molar ratio, the consumption control of added ethyl pyruvate, 1-bromo-4-chlorobutane and sodium hydride is: ethyl pyruvate: 1-bromo-4-chloro Butane: sodium hydride = 1:1:2. After adding 1-bromo-4-chlorobutane, keep the temperature of the mixed solvent at -4°C and react for 12 hours; after the reaction is complete, filter the reaction product and distill the filtrate under reduced pressure (removal of solvent), distillation and purification, that is, 7-chloro-2-oxoheptanoic acid ethyl ester. The yield is 98%.
[0028] Ethyl 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com