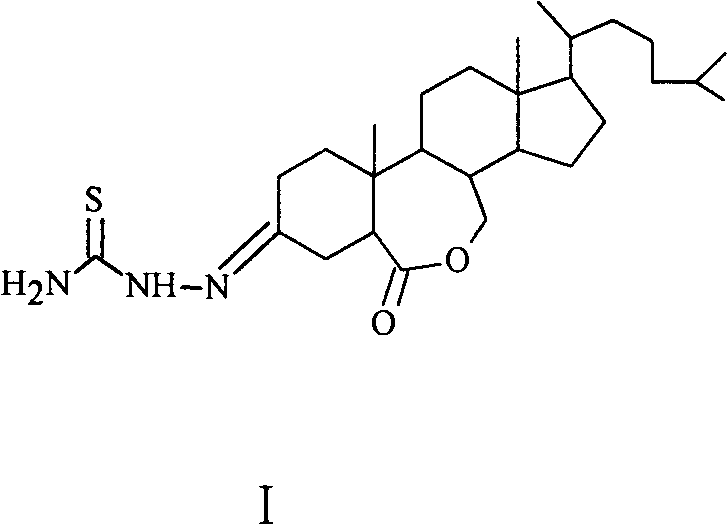
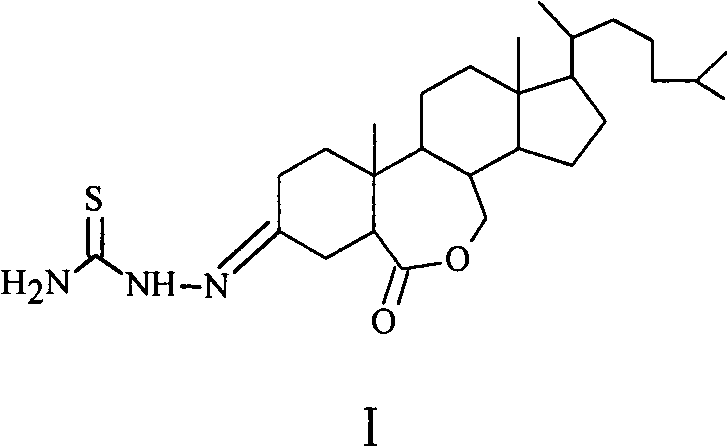
Preparation method of 6-oxo-7-oxa-b-homo-cholesta-3-thionylhydrazone compound and its application in antitumor drugs
A technology of -b-homo- and thionylhydrazone, applied in antineoplastic drugs, steroids, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of 6-oxo-7-oxa-B-Homo-cholesta-3-thionylhydrazone (6)
[0017] Step 1: Preparation of intermediate product (2)
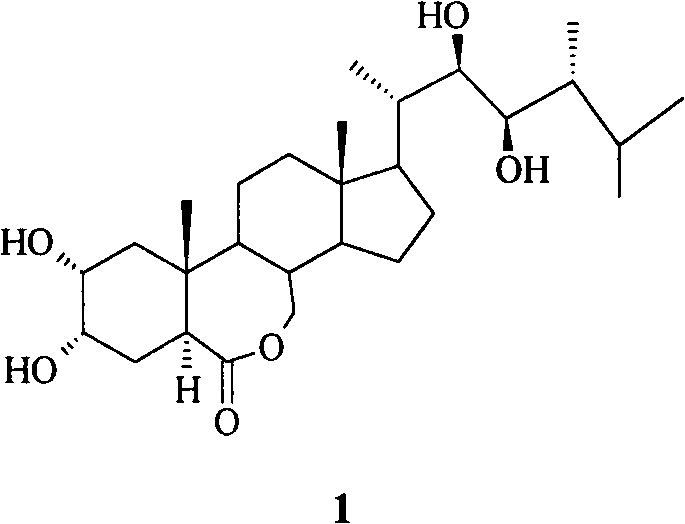
[0018] Add 2.000g (4.09mmol) of cholesterol to the 100mL reaction bottle, and use 80mL CH 2 Cl 2 After dissolving, 5.550 g of pyridinium chlorochromate (PCC) was added at one time, the solution turned black rapidly, and the reaction was carried out with stirring at room temperature. TLC monitoring (ethyl acetate:petroleum ether=1:4), there was no raw material point after 28 hours of reaction, and the reaction was stopped. The reactant was poured into a short column of silica gel, eluted with ethyl acetate, the solvent was distilled off under reduced pressure, and the crude product was separated by 200-300 mesh silica gel column chromatography (eluent: ethyl acetate:petroleum ether=1:6), 1.839 g of yellowish solid 2 was obtained with a yield of 89%. The structure of the product was confirmed by IR, NMR and MS analysis.
[0019] Step 2: Prepara...
Embodiment 2
[0028] This example uses the 6-oxo-7-oxa-B-Homo-cholesta-3--thionylhydrazone compound of the present invention to test the activity of inhibiting tumor cell growth and proliferation on certain tumor cells Test Results.
[0029] Select 6-oxo-7-oxa-B-Homo-cholesta-3--thionylhydrazone compound test of the present invention to human liver cancer cell line (7404), cervical cancer cell line (HeLa), gastric cancer Cytotoxicity of cell line (7901), gastric cancer cell line (MGC-803), lung cancer cell line (SPC-A), and liver cancer cell line (HEPG-2). In vitro cytotoxicity assays were performed using the MTT method. Add different concentrations of 6-oxo-7-oxa-B-Homo-cholesta-3-thionylhydrazone compound to the logarithmic growth phase cells cultured in the 96-well plate, and carry out 3 parallel experiments simultaneously, compared with the control group. After cultivating for 72 hours, add MTT, measure its absorbance, and calculate the concentration of the compound when inhibiting t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com