Novel Skin Cream Using Sodium Fusidate, Antifungals, and Steroids
A technology of sodium fusidate and fusidic acid, applied in the field of bacterial skin infection and inflammation, can solve the problems of stability, heat instability, roughness, uneven particle size, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
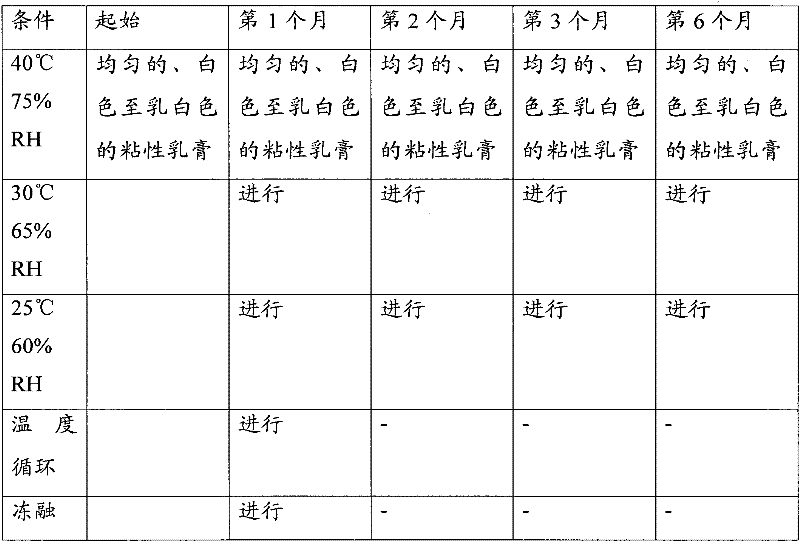
[0598] 1. A novel skin cream comprising at least one corticosteroid, at least one antifungal agent, and fusidic acid prepared in situ using sodium fusidate in an oxygen-free environment, wherein said cream Including fusidic acid made by in situ conversion of sodium fusidate and a cream base comprising at least one of the following: preservatives; primary and secondary emulsifiers; waxy materials ; a co-solvent; an acid; and water, preferably purified water.
[0599] 2. The novel skin cream according to item 1, wherein the corticosteroid is present in an amount ranging from about 0.001% (w / w) to about 5% (w / w), preferably from about 0.005% (w / w) to about 2.00% (w / w), and most preferably about 0.05% (w / w) to 1.0% (w / w),
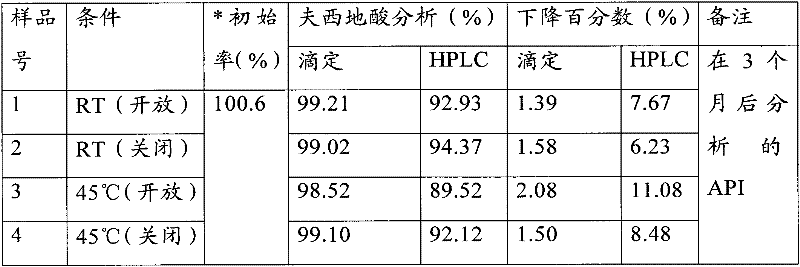
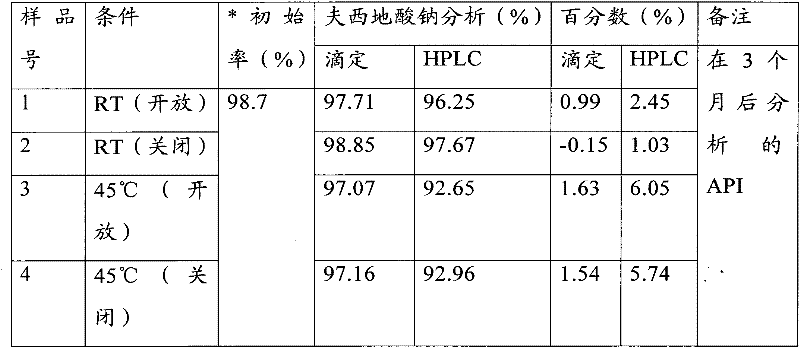
[0600]Described antifungal drug is with about 0.01% (w / w) to about 10% (w / w), preferably about 0.1% (w / w) to about 5.00% (w / w), and most preferably about 1% ( w / w) to 2.0% (w / w), and the fusidic acid is added in an amount of about 0.1% (w / w) to about 25% (w / w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com