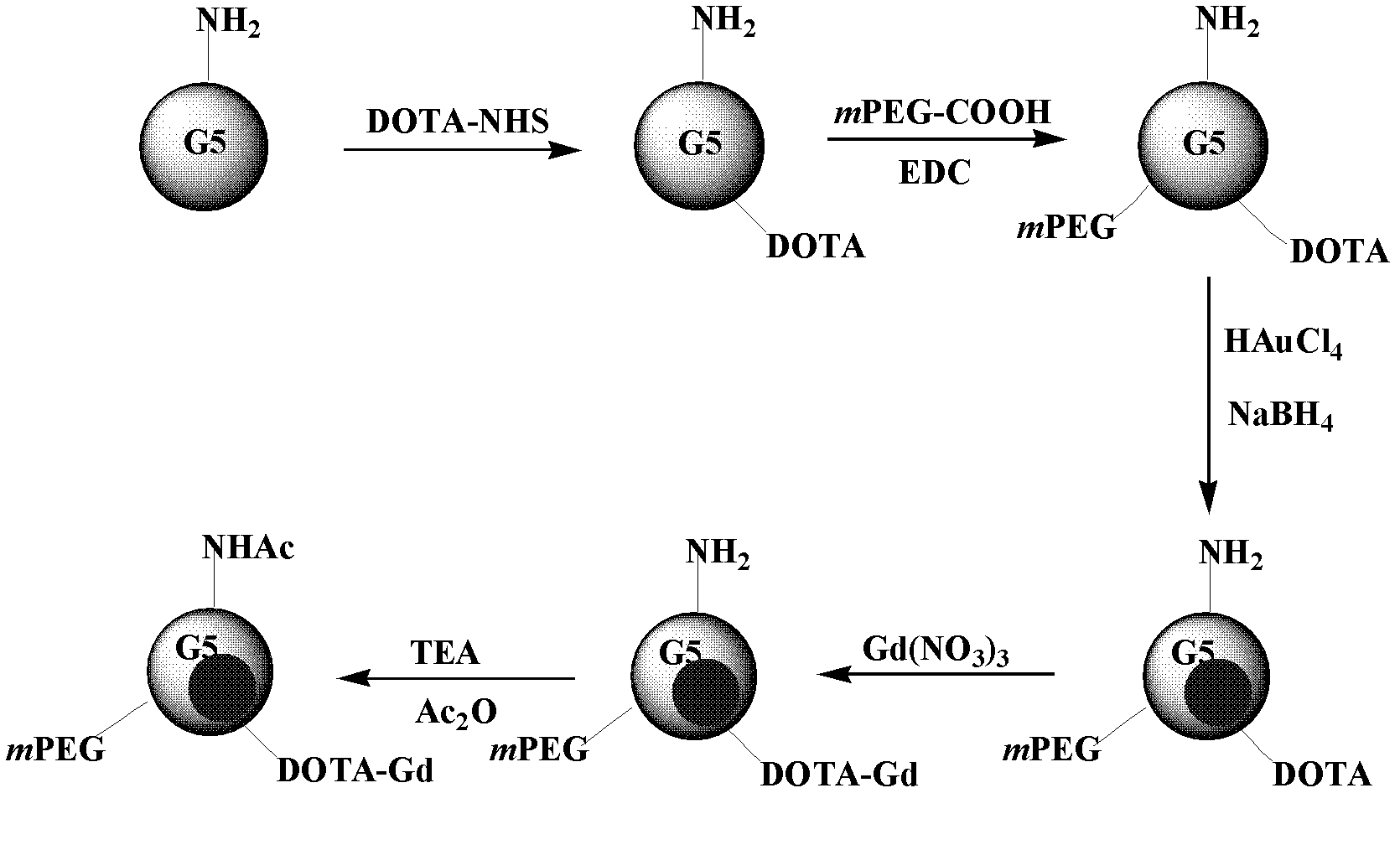
Preparation of CT/MRI dual-modality imaging contrast agent based on dendrimer
A dendritic macromolecule, dual-mode imaging technology, applied in the preparation of X-ray contrast agents, preparations for in vivo tests, pharmaceutical formulations, etc., can solve problems such as the preparation and application of CT/MRI contrast agents that have not been found, and achieve Good MR imaging performance, display signal enhancement, good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] (1) Dissolve G5PAMAM dendrimer with a dry weight of 22.00 mg in 10 mL of DMSO, and add DOTA-NHS with a dry weight of 6.44 mg dissolved in 4 mL of DMSO solution drop by drop while stirring, wherein DOTA-NHS: dendrimer The molecular molar ratio is 10:1, and the reaction takes 24 hours. Add 2 mL of EDC in DMSO (17.2 mg / mL) to 4 mL of mPEG-COOH in DMSO (8.55 mg / mL), stir for 3 hours and add dropwise to the reaction between G5PAMAM dendrimer and DOTA-NHS solution, stirred for 72 hours.
[0062] (2) Take 10mL of the reaction solution in step (1), add 10mL DMSO and 10mL water, stir and mix well, then add 2.29mL HAuCl 4 Aqueous solution (20mg / mL) was mixed, stirred at room temperature for 30min, the solution turned light yellow, then added 1.236mL of 20mg / mL NaBH 4 Solution (H 2 O:CH 3 OH (volume ratio) = 2:1), the solution turned dark red instantly, and stirred and reacted at room temperature for 3h;
[0063] (3) Add 0.535mL Gd(NO 3 ) 3 ·6H 2 Aqueous solution of O (12 ...
Embodiment 2
[0066] (1) Dissolve G5PAMAM dendrimers with a dry weight of 11.00 mg in 5 mL of DMSO, and add DOTA-NHS with a dry weight of 3.22 mg dissolved in 4 mL of DMSO solution drop by drop while stirring, wherein DOTA-NHS: dendrimer The molar ratio of the macromolecules was 10:1, and the reaction was carried out for 18 hours. In 2 mL of mPEG-COOH in DMSO (8.55 mg / mL), add 1 mL of EDC in DMSO (17.2 mg / mL), stir for 3 hours and then add dropwise to the reaction between G5PAMAM dendrimer and DOTA-NHS solution, stirred for 72 hours.
[0067] (2) Take 6mL of the reaction solution in step (1), add 10mL DMSO and 10mL water, stir and mix well, then add 115mL HAuCl 4 Aqueous solution (20mg / mL) was mixed, stirred at room temperature for 20min, the solution turned light yellow, then added 0.618mL of 20mg / mL NaBH 4 Solution (H 2 O:CH 3 OH (volume ratio) = 2:1), the solution turned dark red instantly, and stirred and reacted at room temperature for 1h;
[0068] (3) Add 0.535mL Gd(NO 3 ) 3 ·6...
Embodiment 3
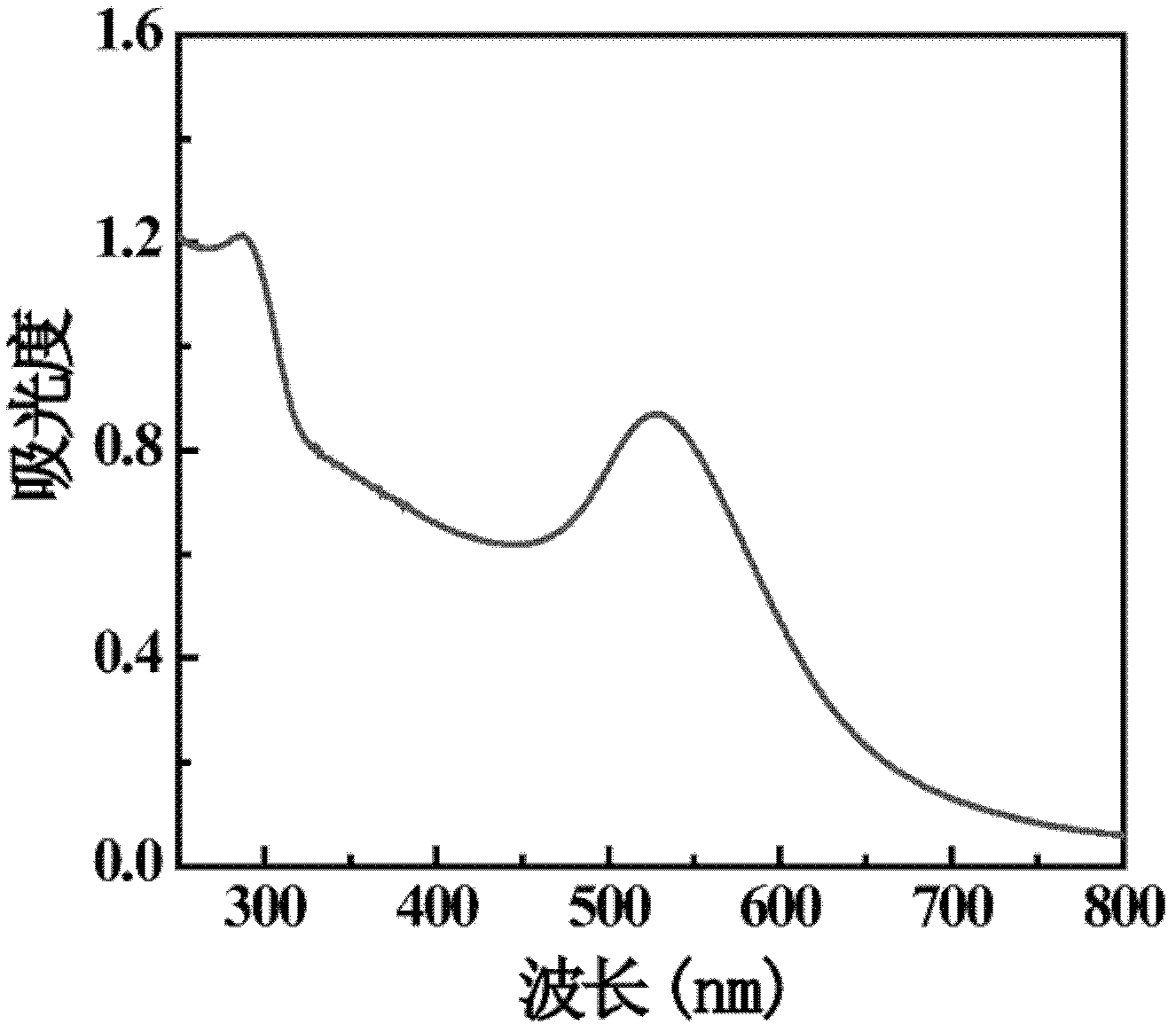
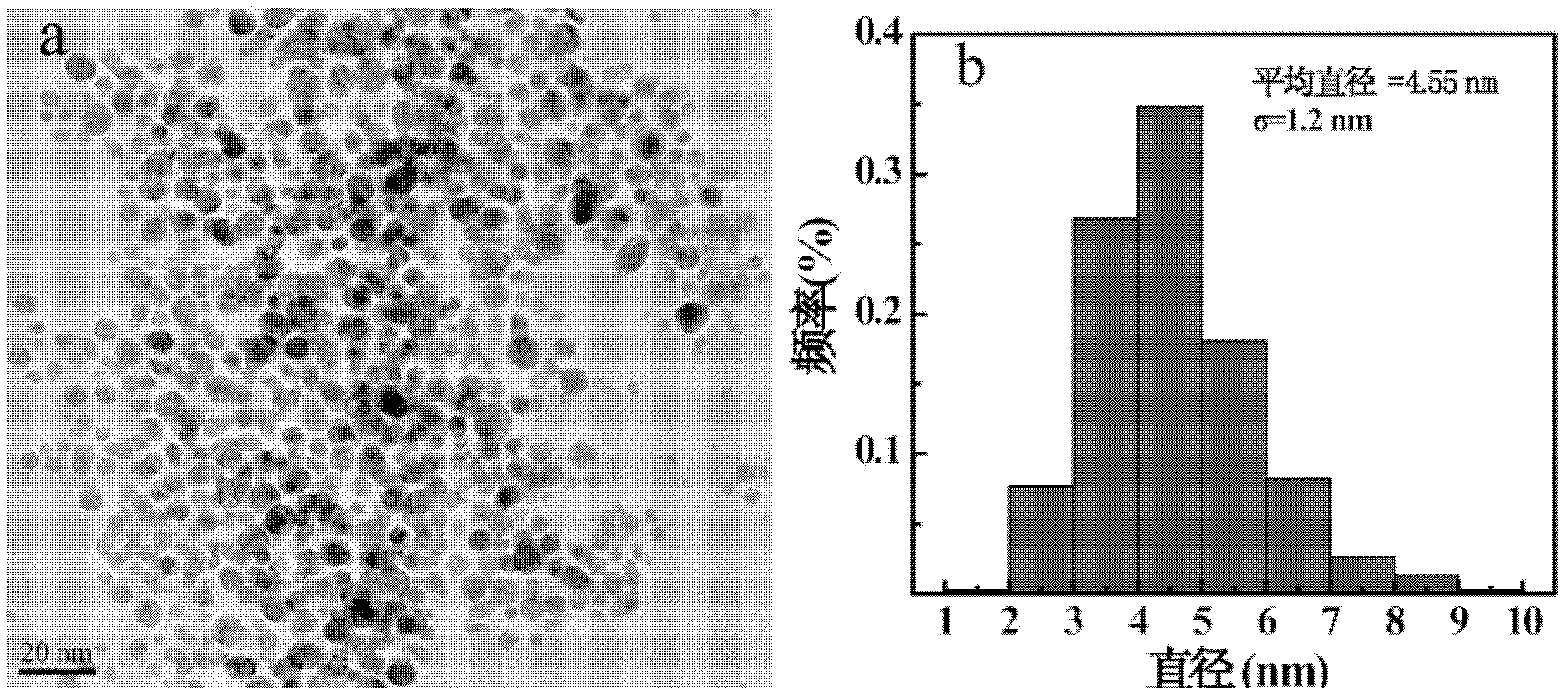
[0071] Check the two kinds of materials {(Au 0 ) 250 -G5-DOTA (GdIII) 10 -PEG 20 -Ac}DENPs and {(Au 0 ) 250 -G5-DOTA 10 -PEG 20 -CT imaging effect of Ac}DENPs. Take 29.88 mg of the sample of Example 1 and dissolve it in 580 μL of PBS buffer to prepare a solution with a gold concentration of 0.1M, and then dilute it to 200 μL each with a gold concentration of 0.08M, 0.05M, 0.03M, 0.02M, and 0.01M. Take 26.51 mg of the sample of Comparative Example 1 and dissolve it in 580 μL of PBS buffer to prepare a solution with a gold concentration of 0.1M, and then dilute it to 200 μL each with a gold concentration of 0.08M, 0.05M, 0.03M, 0.02M, and 0.01M. The HU value of each sample was tested with a CT tester and the linear relationship between X-ray attenuation and gold concentration was obtained. attached Figure 4 (a) is the CT picture of the sample (where 1 is {(Au 0 ) 250 -G5-DOTA (GdIII) 10 -PEG 20 -Ac}DENPs and 2 are {(Au 0 ) 250 -G5-DOTA 10 -PEG 20 -Ac}DENPs), wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com