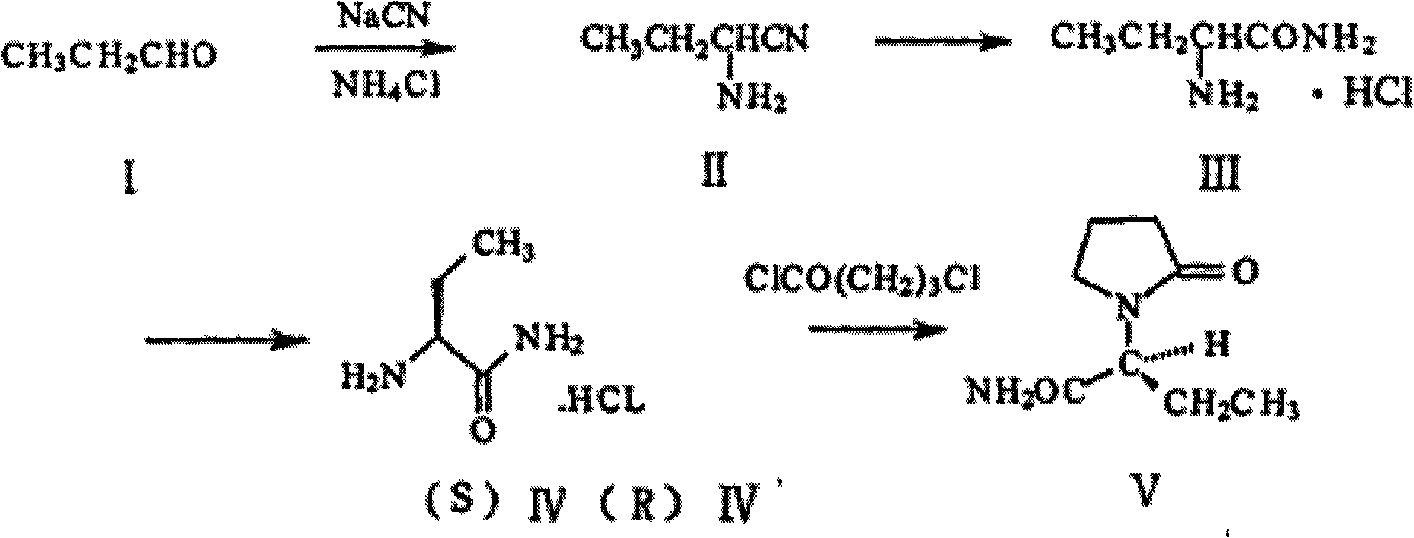
A kind of process improvement method of preparing 2-aminobutyronitrile
A kind of aminobutane cyanide and pressure control technology, applied in the preparation of carboxylic acid nitrile, the preparation of carboxylic acid amide, chemical instruments and methods, etc., can solve the problem of low yield, improve yield, make full use of, and simplify separation and purification operations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In the reactor of 1000mL, add successively the sodium cyanide aqueous solution that is made into by the water of 73.5g (1.5mol) sodium cyanide, 171g, 60.2g (1.125mol) ammonium chloride; Pass into ammonia gas under stirring, ice-water bath cooling, The temperature of the reaction system was maintained within 10°C, and ammonia gas was passed for 2 hours; after passing through, 0.5g of benzyltriethylammonium chloride was added, and while the reaction temperature was maintained, 43.5g (0.75mol) of n-propionaldehyde, 1.5 The dropwise addition was completed within 1 hour; after continuing to stir for 30 minutes, feed ammonia gas to make the pressure 0.3 MPa, rise to room temperature (20-25° C.) and react for 20 hours. After the reaction was completed, extract with dichloromethane (100mL×3), combine the organic phases, wash the organic phase once with saturated brine, dry over anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure to obtain 49g of a yell...
Embodiment 2
[0026] The water phase after extraction and separation of Example 1 was placed in a 1000mL reaction kettle, and 40.1g (0.75mol) of ammonium chloride and 36.8g (0.75mol) of solid sodium cyanide were added successively, ammonia gas was introduced under stirring, and cooled in an ice-water bath , the temperature of the reaction system was maintained within 10°C, and the ammonia gas was passed for 2 hours; after the pass was completed, and the reaction temperature was maintained, 43.5g (0.75mol) of n-propionaldehyde was slowly added dropwise, and the dropwise addition was completed within 1.5 hours; after continuing to stir for 30 minutes Feed ammonia gas to make the pressure 0.3 MPa, rise to room temperature (20-25° C.) and react for 20 hours. After the reaction was completed, extract with dichloromethane (100mL×3), combine the organic phases, wash the organic phase once with saturated brine, dry over anhydrous sodium sulfate, filter, evaporate the solvent under reduced pressure t...
Embodiment 3
[0028] The water phase after extraction and separation of Example 2 was placed in a 1000mL reaction kettle, 40.1g (0.75mol) of ammonium chloride and 36.8g (0.75mol) of solid sodium cyanide were added in sequence, ammonia gas was introduced under stirring, and cooled in an ice-water bath , the temperature of the reaction system was maintained within 10°C, and the ammonia gas was passed for 2 hours; after the pass was completed, and the reaction temperature was maintained, 43.5g (0.75mol) of n-propionaldehyde was slowly added dropwise, and the dropwise addition was completed within 1.5 hours; after continuing to stir for 30 minutes Feed ammonia gas to make the pressure 0.3 MPa, rise to room temperature (20-25° C.) and react for 20 hours. After the reaction is complete, extract with dichloromethane (100mL×3), combine the organic phases, wash the organic phase once with saturated brine, dry over anhydrous sodium sulfate, filter, and evaporate the solvent under reduced pressure to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com