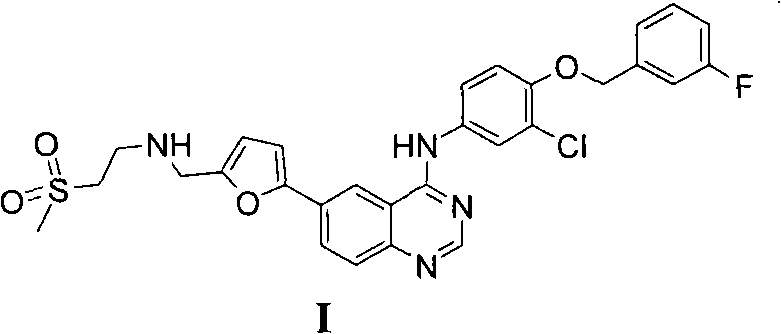
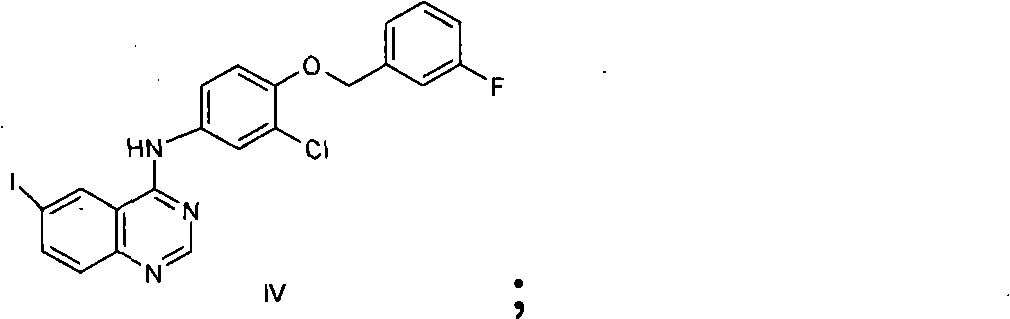
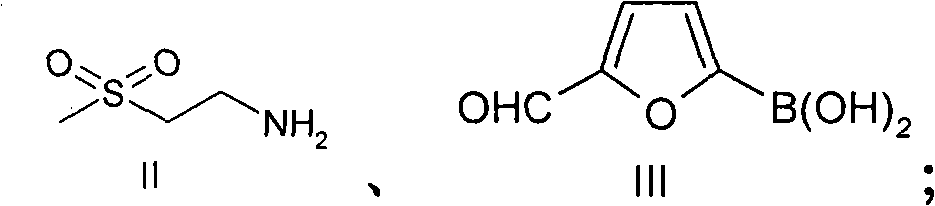New preparation method of lapatinib
A compound and reaction technology applied in the field of synthesis of small molecule targeted drug lapatinib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0107] In the preparation method of the present invention, the reducing agent is selected from sodium triacetoxyborohydride, sodium borohydride and potassium borohydride, among which sodium triacetoxyborohydride is preferred.
[0108] In the preparation method of the present invention, the reaction temperature used to generate transition intermediate 1 is 0-80°C; the reaction temperature used to generate transition intermediate 2 is 40°C-130°C; the imine is reduced to obtain lapatidine The reaction temperature used by Nietzsche is -20~40℃.
[0109] In the preparation method of lapatinib described in the present invention, each starting material can make full use of each functional group on the chemical structure during the reaction process, and react to obtain the desired final product. Specifically, the amino group in compound II reacts with the aldehyde group in compound III to form an imine, which avoids the instability caused by the oxidation of the aldehyde group at high ...
Embodiment 1
[0117] Add 10L N,N-dimethylformamide to the 20L reactor, then add the starting material compound II 352g (1.485mol), compound III 194g (1.386mol), compound IV 500g (0.990mol), triethylamine 1500g, (dppf)PdCl 2 10g, stirred evenly, under nitrogen protection, stirred at 25 ° C, TLC analysis of raw material compound III after the reaction was complete, the temperature was raised to reflux reaction, TLC analysis of the raw material compound IV after the reaction was complete, cooled to room temperature, added sodium triacetoxyborohydride 630g (2.97 mol), stirring reaction at room temperature, TLC analysis transition intermediate 2 after the complete reaction, suction filtration, adding 20L dichloromethane to the filtrate, washing with 10L 1N sodium hydroxide solution, washing with 20L saturated sodium chloride solution, drying over anhydrous sodium sulfate , suction filtration, the filtrate was added under stirring and 750g (3.94mol) p-toluenesulfonic acid was added, crystallized ...
Embodiment 2
[0119] Add 10L ethanol to the 20L reactor, then add the starting material compound II 352g (1.485mol), compound III 194g (1.386mol), compound IV500g (0.990mol), triethylamine 1500g, (dppf)PdCl 2 10g, stirred evenly, under nitrogen protection, stirred at room temperature, after TLC analysis raw material III reacted completely, was heated to reflux reaction, after TLC analyzed raw material IV reacted completely, cooled to room temperature, added triacetoxy sodium borohydride 630g (2.97mol), Stir the reaction at room temperature, TLC analysis transition intermediate 2 After the reaction is complete, filter with suction, add 750g (3.94mol) p-toluenesulfonic acid to the filtrate under stirring, crystallize at room temperature, filter with suction, and dry in the air to obtain lapatinib p-toluenesulfonic acid Salt 725g, yield 79.2%, HPLC purity 98.35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com