HPLC (High Performance Liquid Chromatography) method for utilizing chiral column to separate, identify and prepare monomer matter from cucurbitacin mixture
A technology of cucurbitacins and mixtures, which is applied in the field of medicine and can solve problems such as ineffective separation of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1 uses Preparation of five kinds of cucurbitacins by AD-H chiral column
[0079] 1 Experimental equipment and chromatographic conditions
[0080] P230 high-pressure constant-flow pump (preparative type), UV228 ultraviolet-visible detector (Dalian Yilite Scientific Instrument Co., Ltd.); Chiralpak AD-H chiral column (preparative type, 10 μm Daicel Chemical Industry Co., Ltd.)
[0081] Mobile phase: n-hexane: isopropanol: ethanol: trifluoroacetic acid (75:15:10:0.1) (v / v)
[0082] Flow rate: 3.0mL / min
[0083] Column temperature: room temperature
[0084] Detection wavelength: 220nm
[0085] Injection volume: 200μL
[0086] 2 Preparation of samples
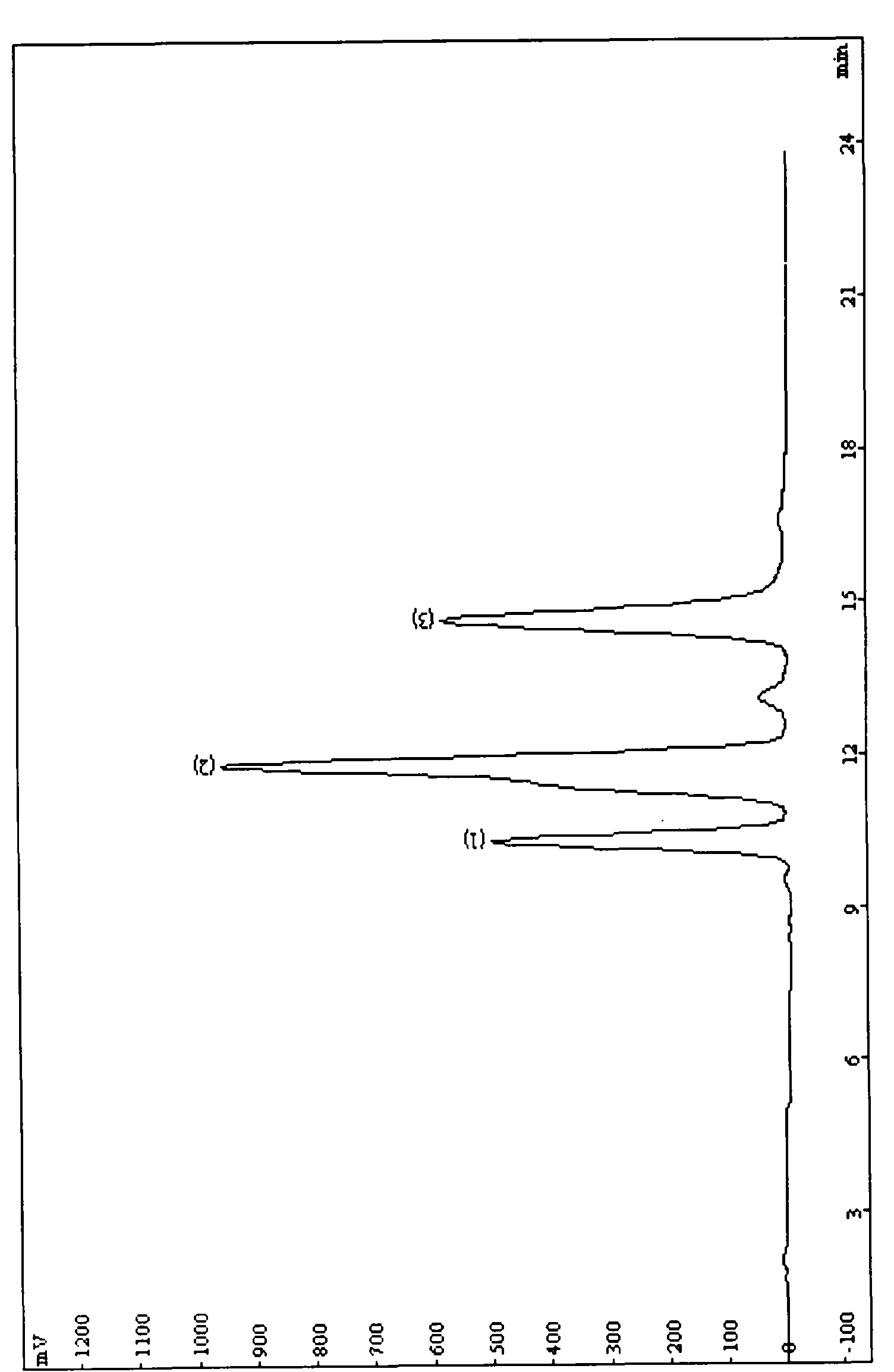
[0087] Take 200 mg of cucurbitacin mixture (containing 15% cucurbitacin B), add 5 mL of ethanol to dissolve it, filter through a 0.45 μm microporous membrane, and inject the sample (separation effect is as follows: figure 2 As shown), the effluent was collected in sections to obtain a solution containing fi...
Embodiment 2
[0112] Example 2 Analytical type under different mobile phases Separation of Cucurbitacin Raw Material by AD-H Chiral Column
[0113] Other operations are the same as embodiment 1, the difference is:
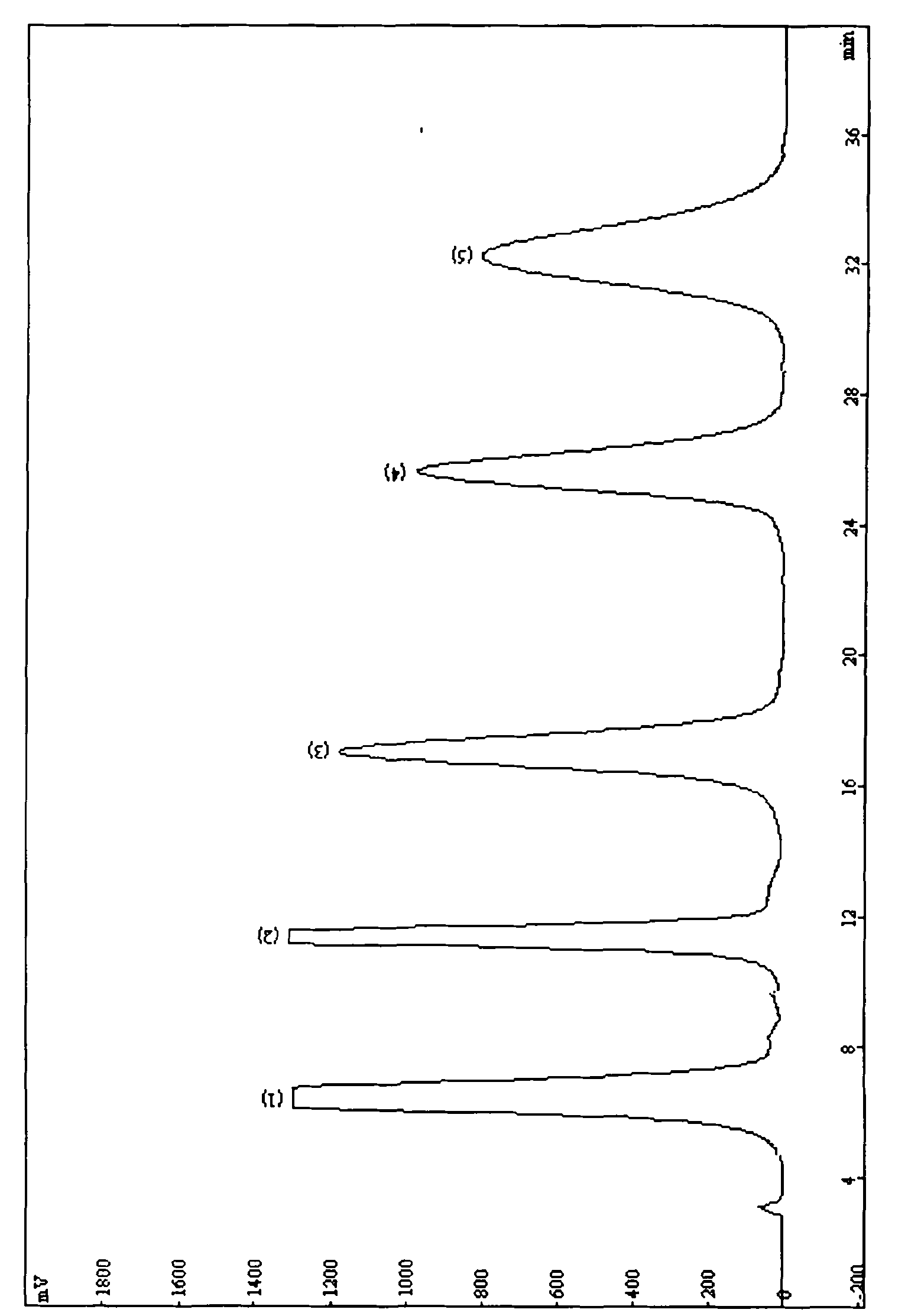
[0114] With volume ratio n-hexane: ethanol: isopropanol: glacial acetic acid=70: 20: 10: 0.1 as the mobile phase cucurbitacin mixture for separation and identification, the separation effect is as follows Figure 4-A mentioned.
[0115] Or volume ratio n-hexane: ethanol: trifluoroacetic acid=70: 30: 0.1 is that mobile phase cucurbitacin mixture is separated and identified, and its separation effect is as follows Figure 4-B mentioned.
[0116] Or with volume ratio normal hexane: isopropanol=80:20 is that mobile relative raw material is separated, and its separation effect is as follows Figure 4-C shown.
Embodiment 3
[0117] Various cucurbitacin monomer content determination methodological investigation in the cucurbitacin mixture of embodiment 3
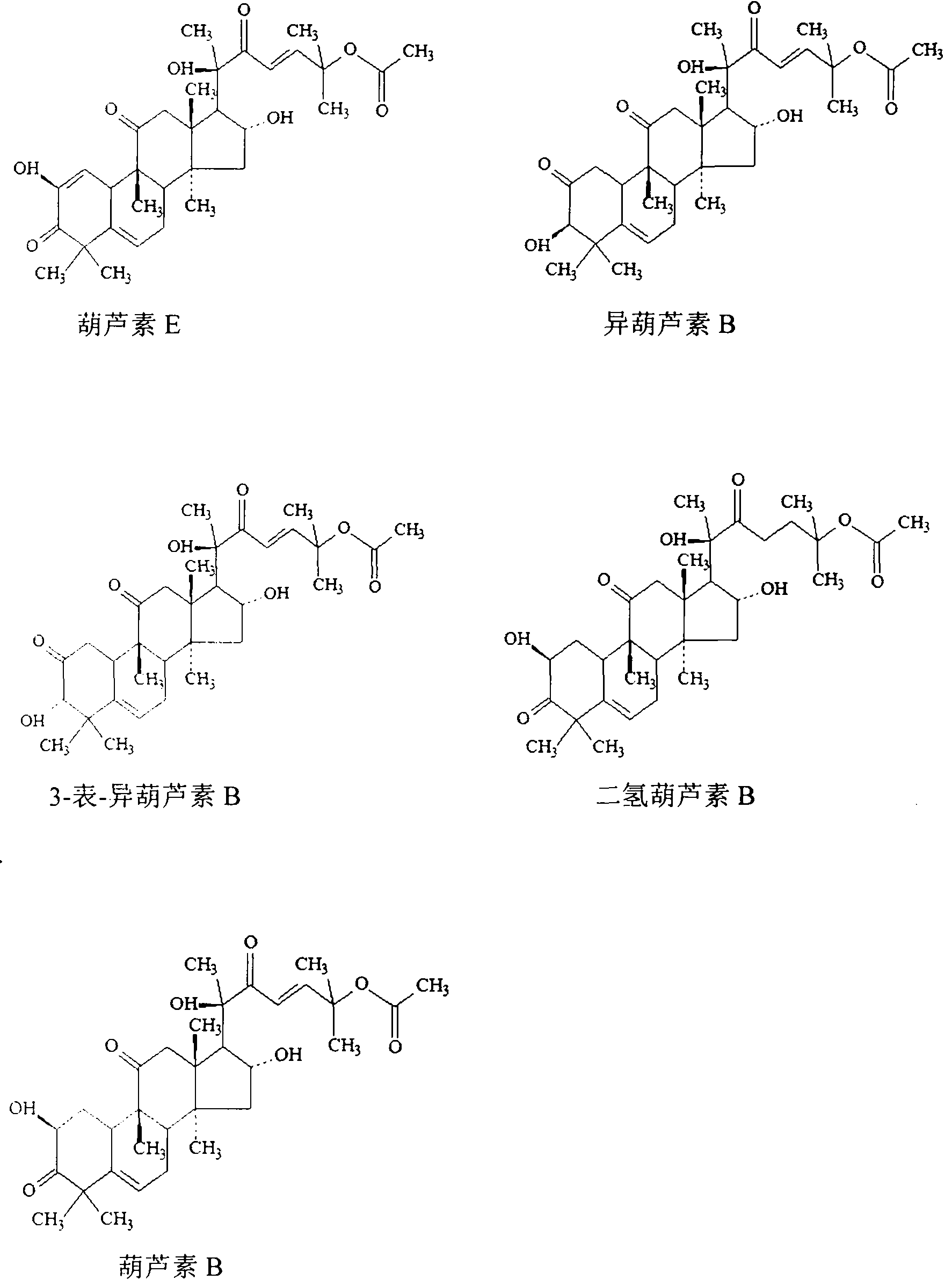
[0118] Standard curve Accurately weigh the appropriate amount of cucurbitacin E, isocucurbitacin B, 3-epi-isocucurbitacin B, dihydrocucurbitacin B and cucurbitacin B reference substances, dissolve and dilute to a concentration of about 40 μg mL with mobile phase -1 (Cucurbitacin E, Isocucurbitacin B, 3-Epi-Isocucurbitacin B), 0.1mg·mL -1 (dihydrocucurbitacin B) and 0.7mg·mL -1 (cucurbitacin B) mixed solution as a control stock solution. Precisely measure 1.0, 3.0, 5.0, 7.0, and 9.0 mL of the reference substance stock solution and put it in a 10 mL measuring bottle, dilute to the mark with mobile phase, shake well, and prepare a series of reference substance solutions. Precisely draw 20 μL each of the series of control solutions, inject them into the liquid chromatograph, and record the peak areas of the chromatographic peaks of the five compone...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| injection volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com