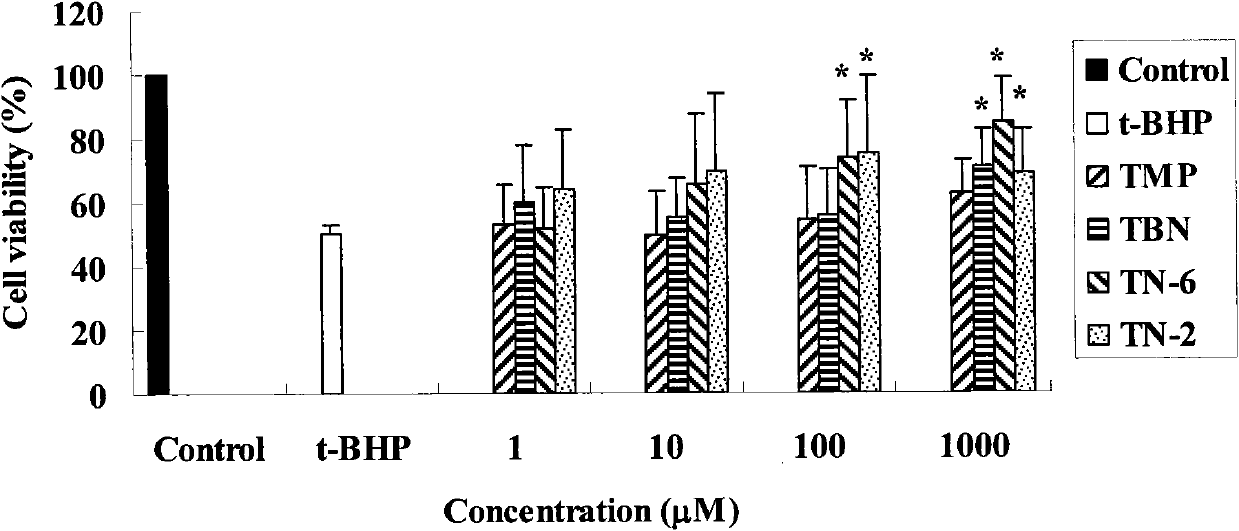
Pyrazine derivative and preparation method as well as application thereof to pharmacy
A technology of pyrazines and derivatives, which is applied in the field of pyrazines and can solve the problems of poor drug efficacy, large toxic and side effects, and weak antioxidant effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
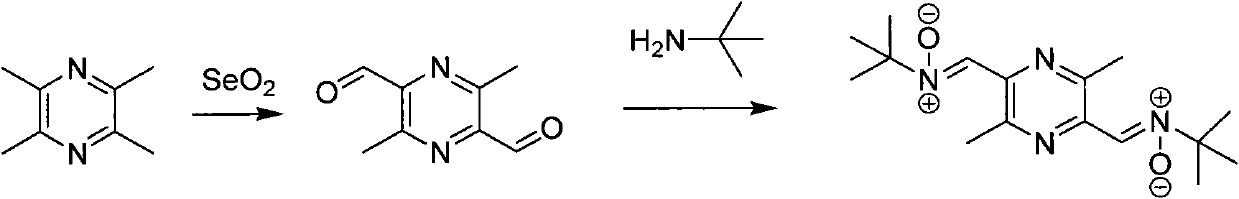
[0084] The synthesis of embodiment 1, TN-2 ( figure 1 )
[0085] Add 200mL of methanol to a 500mL three-necked flask, add 2.0g (0.012mol) of 3,6-dimethyl-2,5-pyrazinedicarbaldehyde, then add 4.3g (0.048mol) of tert-butylhydroxylamine, and heat to reflux 3h. Separation by column chromatography (ethyl acetate 100%) gave 1.0 g of light yellow solid compound TN-2. Yield 26.8%, mp: 198-201°C. 1 H NMR (CDCl 3 ): 1.61(s, 18H), 2.48(s, 3H), 2.50(s, 3H), 7.83(s, 2H); ESI-MS: 307[M+H] + , 329[M+Na] + ;Anal.(C 12 H 19 N 3 O) C.H.N; found C 62.52%, H 8.73%, N 18.19%; requires: C, 65.13; H, 8.65; N, 18.99.
[0086]
Embodiment 2
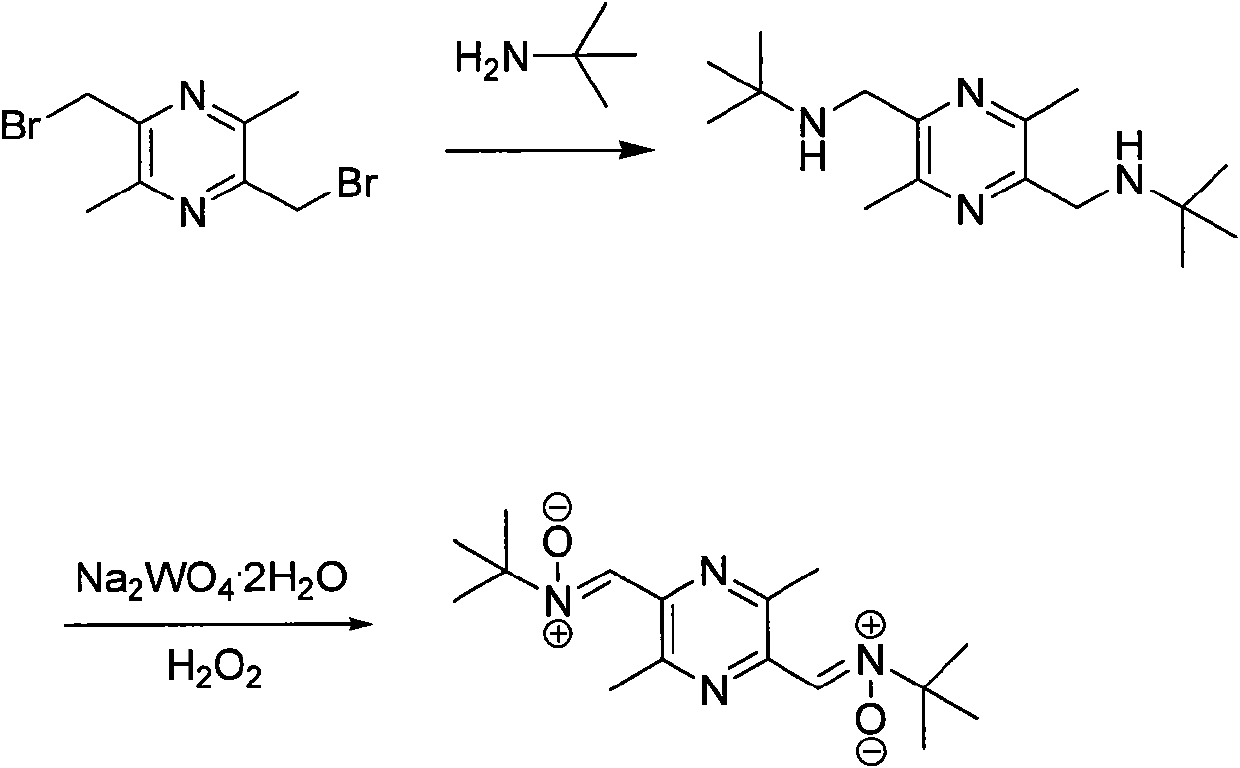
[0087] The synthesis of embodiment 2, TN-2 ( figure 2 )
[0088] Add 5.6g (0.02mol) of 2,5-di-tert-butylaminomethyl-3,6-dimethylpyrazine into a 250mL round bottom flask, add an appropriate amount of methanol, and add 1.64g (0.005mol) of Na 2 WO 4 · 2H 2 O, 30% H 2 O 2 10mL, stirred at room temperature for 2h. Filter, evaporate methanol, add saturated Na 2 S 2 O 3 , extracted with ethyl acetate, and evaporated most of the ethyl acetate. After separation by column chromatography (ethyl acetate 100%), a white solid TN-21.97g was obtained with a yield of 32%, and the detection data was the same as above.
Embodiment 3
[0089] The synthesis of embodiment 3, TN-4
[0090] Add 2.88g (0.02mol) of 2-methylquinoxaline in the three-necked bottle of 250mL, 20mg benzoyl peroxide, add 80mL CCl 4 , Reflux at 70°C for 10h. After cooling and filtering, the crude product of 2-bromomethylquinoxaline was obtained. The compound was not separated, and an excess of tert-butylamine was added, and stirred at room temperature for 3h. 1.25 mg of 5-tert-butylaminomethylquinoxaline was obtained with a yield of 29.1%.
[0091] Add 60mL methanol to the 670mg (0.006mol) compound obtained above, Na 2 WO 4 · 2H 2 O 0.18g, 30%H 2 O 21.75mL, react at room temperature for 2.5h. Separation by column chromatography (ethyl acetate:petroleum ether=4:1) gave the light yellow compound TN-3, 460mg, yield 35.9%. 1 HNMR (CDCl 3 ): 1.70(s, 9H), 7.77(m, 2H), 8.03(m, 2H), 8.14(s, 1H), 10.49(s, 1H); ESI-MS: 230[M+H] + ;Anal.(C 13 H 15 N 3 O) C.H.N; found C 67.80%, H 6.90%, N 17.86%; requires: C, 68.10; H, 6.59; N, 18.33. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com