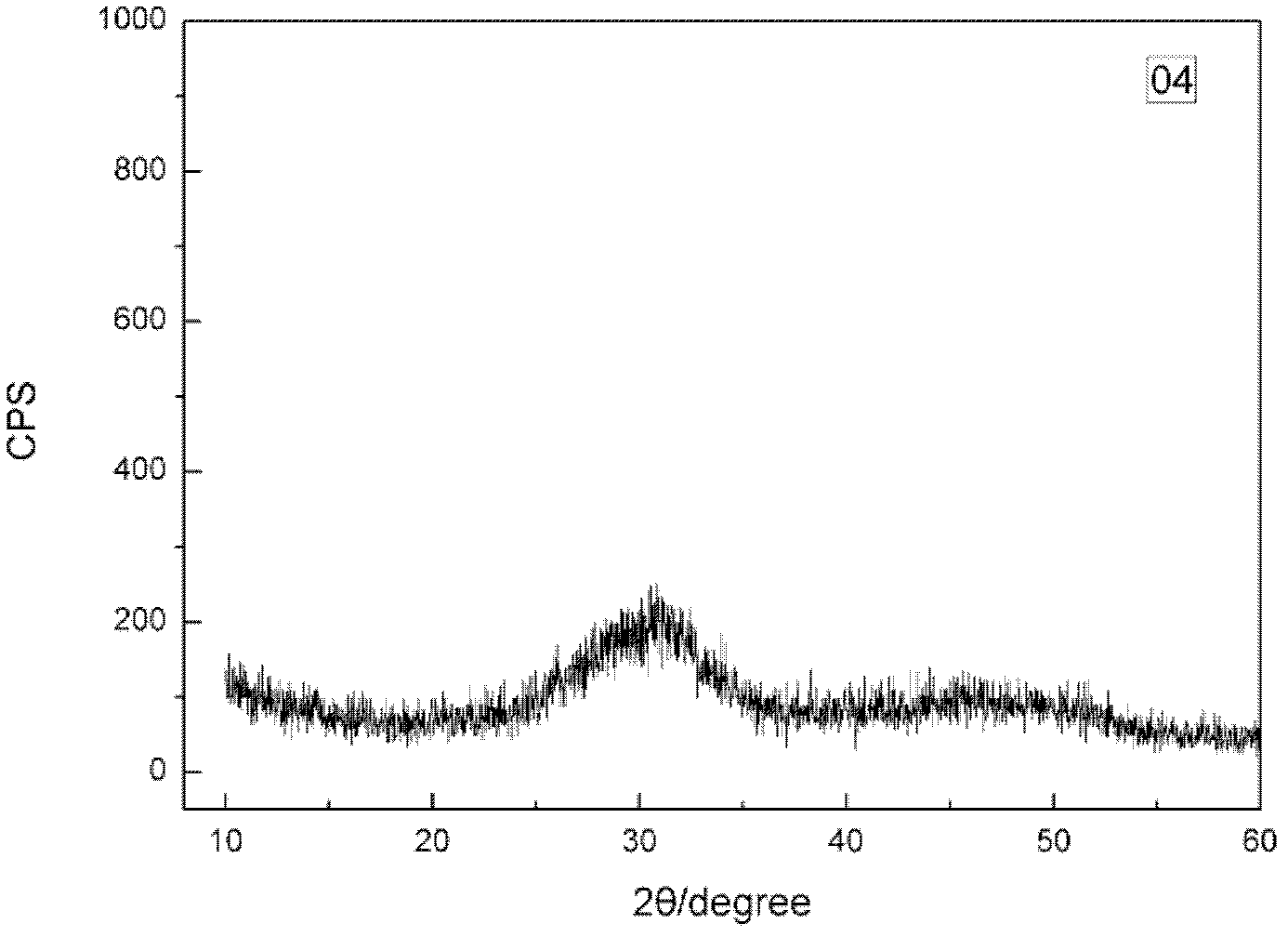
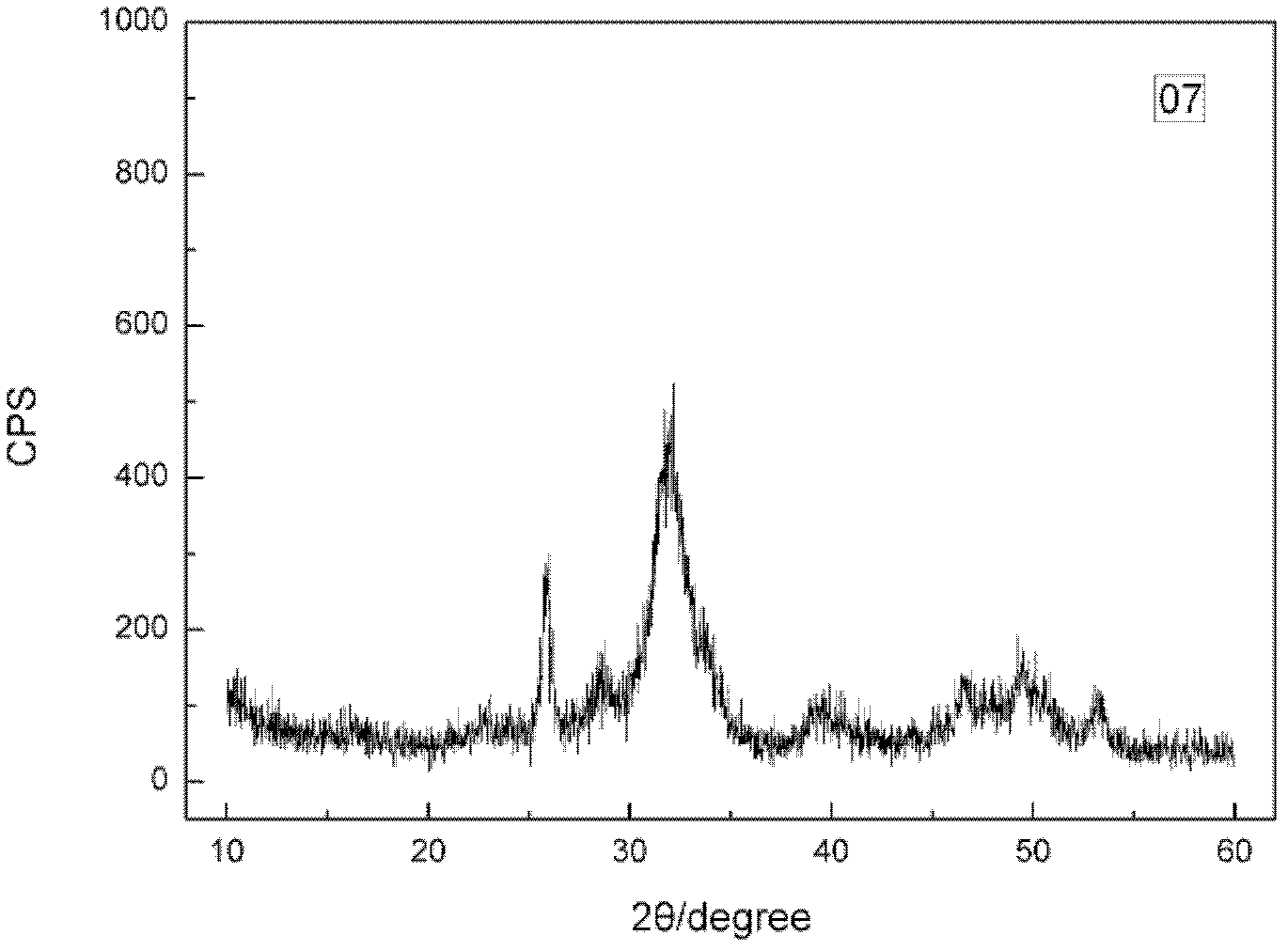
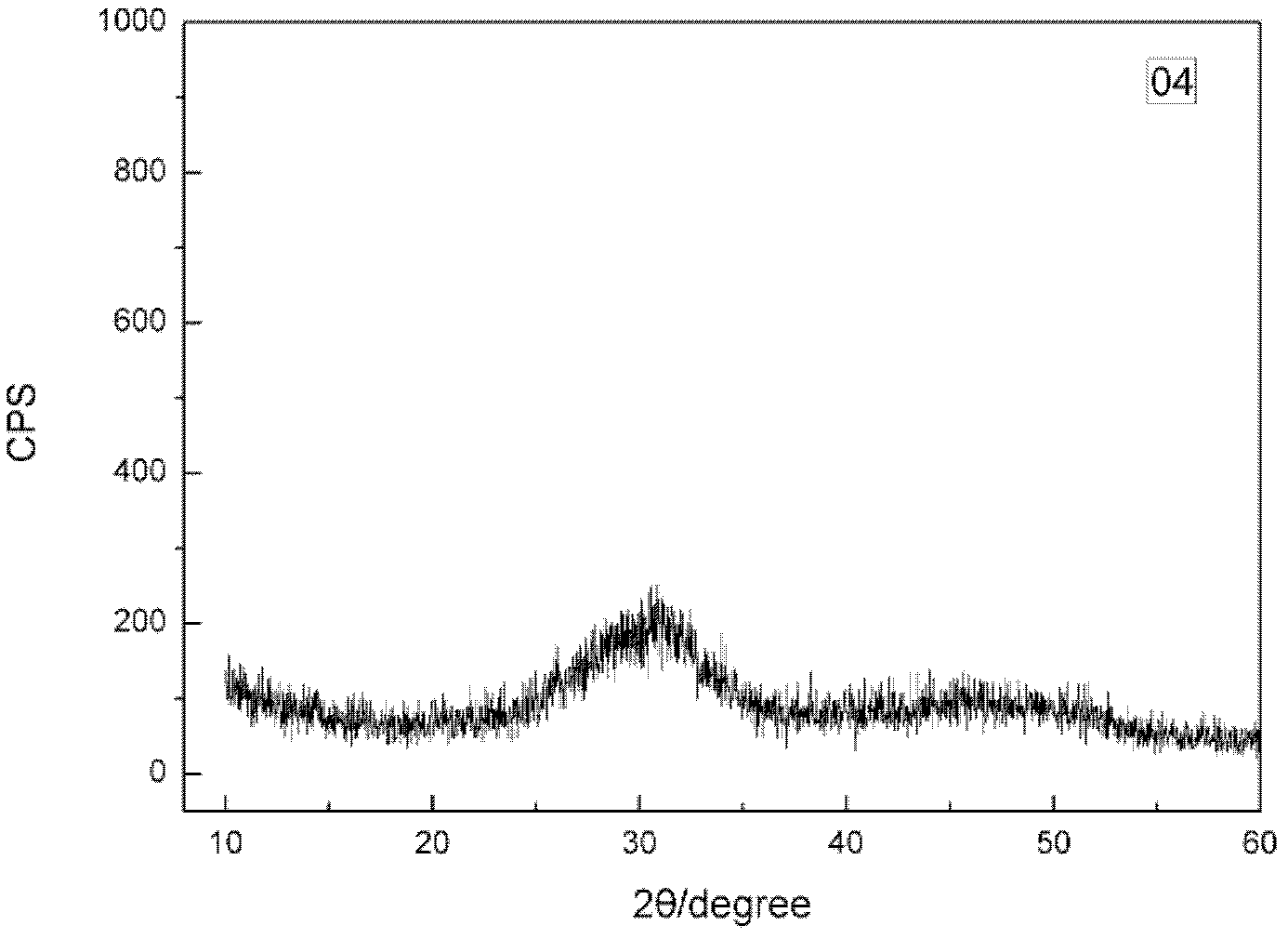
Synthesizing method of amorphous calcium phosphate
A synthesis method and technology of crystalline calcium phosphate, applied in phosphorus compounds, chemical instruments and methods, non-metallic elements, etc., to achieve the effects of simple and easy process operation, simple equipment and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A kind of synthetic method of ACP, the steps are as follows:
[0027] (1) Take by weighing calcium nitrate tetrahydrate (Ca(NO 3 ) 2 4H 2 O) 5.9g, diammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) 1.98g; then the weighed Ca(NO 3 ) 2 4H 2 O is dissolved in 250ml absolute ethanol solution and is made into solution A, the (NH 4 ) 2 HPO 4 Dissolve in a mixed solution of 100ml of absolute ethanol and 200ml of deionized water to form solution B; stir solution A at a constant temperature at 0°C (ice-water mixture) and solution B at room temperature;
[0028] (2) The concentration of calcium ions in solution A is 0.1mol / L, and the concentration of phosphate ions in solution B is 0.01mol / L; add ammonia water in solution A to adjust the pH to 11.5 to obtain solution C, add ammonia water in solution B Adjust the pH to 11.5 to obtain solution D;
[0029] (3) Place solution C in an ultrasonic environment at 0°C (in an ultrasonic generator, using a water bath, the ultrason...
Embodiment 2
[0033] (1) Take by weighing calcium nitrate tetrahydrate (Ca(NO 3 ) 2 4H 2 O) 5.9g, diammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) 1.98g; then the weighed Ca(NO 3 ) 2 4H 2 O is dissolved in 100ml absolute ethanol solution and is made into solution A, the (NH 4 ) 2 HPO 4 Dissolve in a mixed solution of 80ml of absolute ethanol and 100ml of deionized water to form solution B; stir solution A at a constant temperature at 0°C (ice-water mixture) and solution B at room temperature;
[0034] (2) The amount of calcium ions in solution A is 0.25mol / L, and the amount of phosphate ions in solution B is 0.083mol / L; add ammonia water in solution A to adjust the pH to 11.5 to obtain solution C, add ammonia water in solution B Adjust the pH to 11.5 to obtain solution D;
[0035] (3) Place solution C in an ultrasonic environment at 0°C (in an ultrasonic generator, using a water bath, the ultrasonic electric power is 250W, and the operating frequency is 40KHZ), and use a separato...
Embodiment 3
[0039] (1) Take by weighing calcium nitrate tetrahydrate (Ca(NO 3 ) 2 4H2 O) 5.9g, diammonium hydrogen phosphate ((NH 4 ) 2 HPO 4 ) 1.98g; then the weighed Ca(NO 3 ) 2 4H 2 O is dissolved in 50ml absolute ethanol solution and is made into solution A, the (NH 4 ) 2 HPO 4 Dissolve in 30ml deionized water to make solution B; stir solution A at constant temperature at 0°C (ice-water mixture) and solution B at room temperature;
[0040] (2) The amount of calcium ions in solution A is 0.5mol / L, and the amount of phosphate ions in solution B is 0.5mol / L; add ammonia water in solution A to adjust the pH to 11.5 to obtain solution C, add ammonia water in solution B Adjust the pH to 11.5 to obtain solution D;
[0041] (3) Place solution C in an ultrasonic environment at 0°C (in an ultrasonic generator, using a water bath, the ultrasonic electric power is 300W, and the operating frequency is 35KHZ), and drop solution D into solution C with a separatory funnel, Stir solution C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com