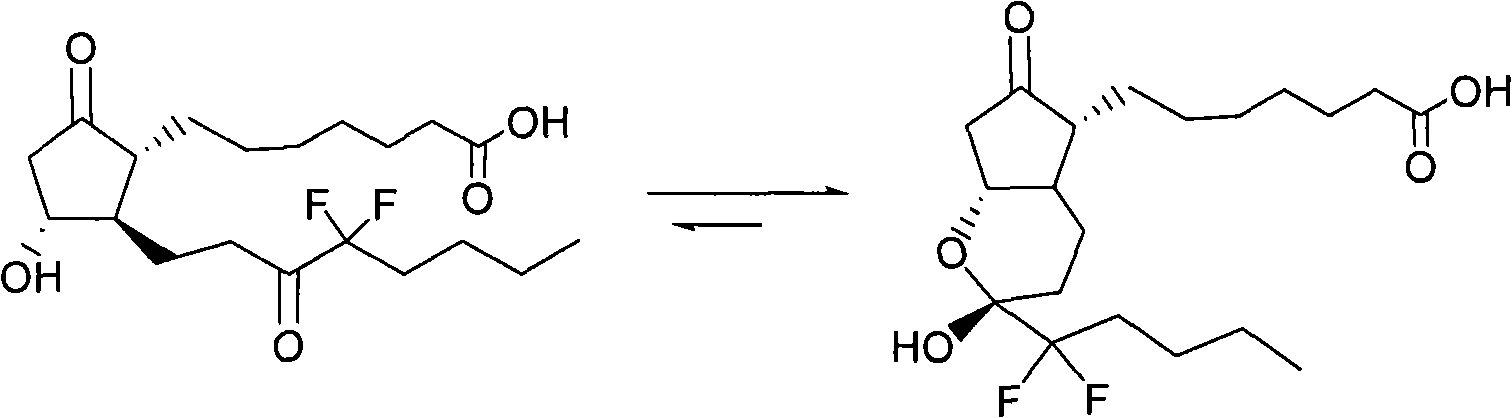
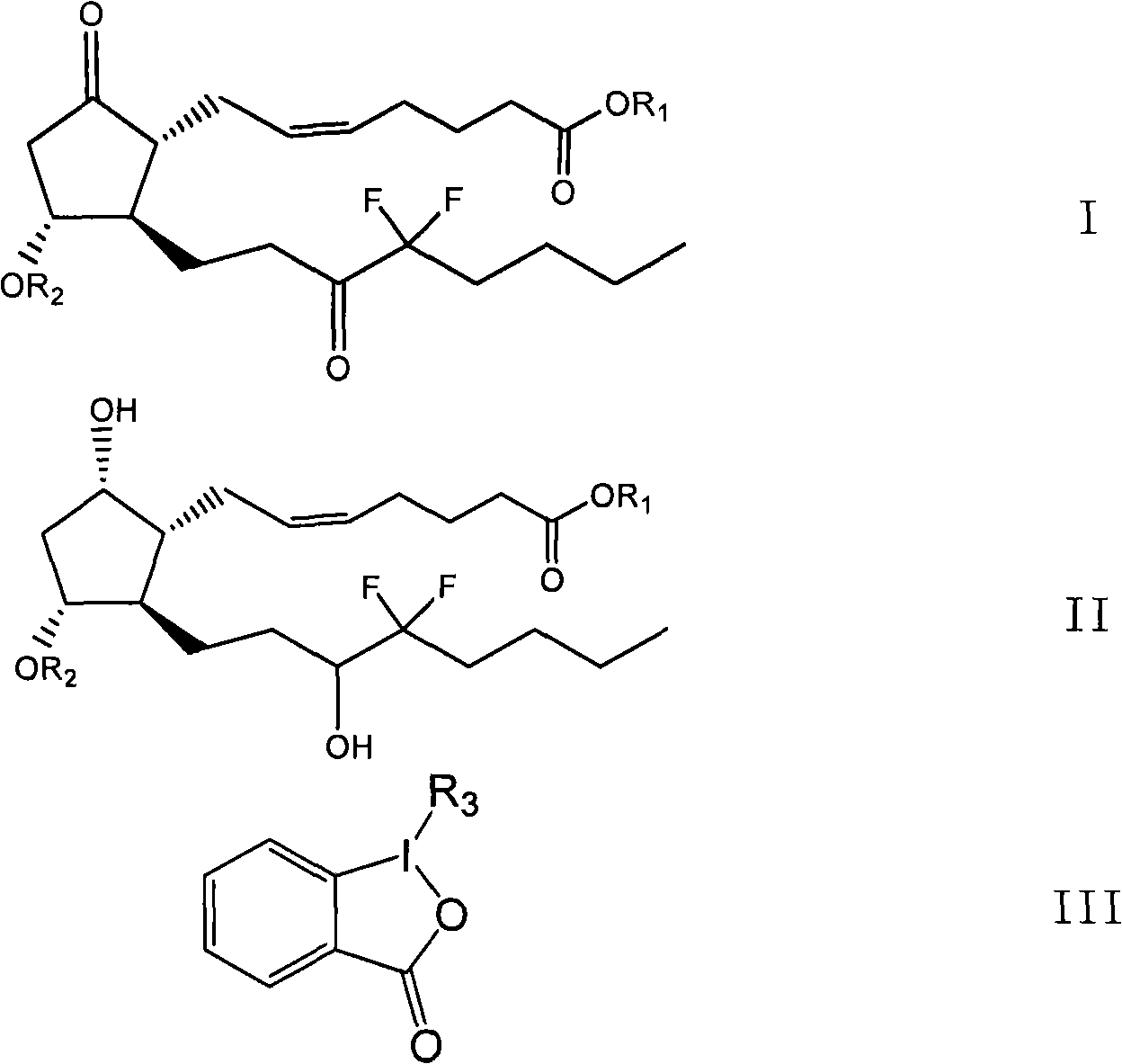
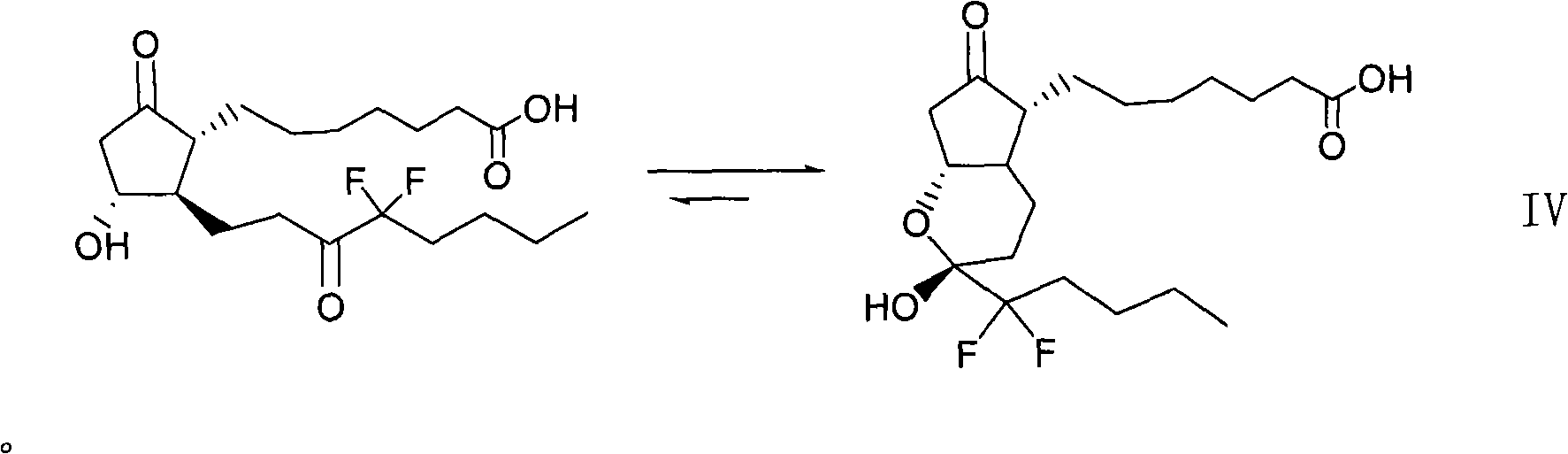
Preparation method for prostaglandin intermediate
A compound and solution technology, which is applied in the field of preparation of prostaglandin intermediates, can solve the problems of violent reaction, environmental pollution, difficult control and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of the compound of formula I provided by the invention comprises the following steps:
[0035] In the first step, the solution 2 containing the compound shown in formula II and the solution 3 containing the compound shown in formula III are mixed at 0-10°C, and then stirred at 0-30°C; the solution 2 or solution 3 contained The solvent is dichloromethane;
[0036] In the second step, after adding an aqueous solution, back-extract 1-3 times with dichloromethane, and combine the dichloromethane layers; and
[0037] In the third step, the obtained dichloromethane layer solution is subjected to silica gel chromatography to obtain the compound shown in formula I.
[0038]
[0039] Preferably, in the first step, the solution 2 containing the compound represented by formula II can be added dropwise at 0-10°C to the solution 3 containing the compound represented by formula III, and then reacted at 0-30°C; or the solution containing After the solution ...
Embodiment 1
[0056] Preparation of 16,16-difluoro-13,14-dihydro-15-carbonyl-11-[(tetrahydro-2H-pyran-2-yl)oxy]-PGE 2 methyl ester 1
[0057]
[0058] Dess-Martin oxidizer (105.7 g) was stirred and dissolved in dichloromethane (1300 ml) at room temperature, N 2Under protection, cool to 0°C, add dropwise 16,16-difluoro-13,14-dihydro-15(R,S)-hydroxy-11-[(tetrahydro-2H-pyran-2-yl)oxy ]-PGF 2 A solution of methyl ester (54.3g) in dichloromethane (340ml) was added dropwise over 20 minutes. Rising to 10°C and stirring for 5h. TLC detects that the reaction is complete.
[0059] A 1.3 L aqueous solution of 377.6 g of sodium thiosulfate pentahydrate and 139.8 g of sodium bicarbonate was slowly added. Stir for 5 min, separate layers, back-extract the water layer with 200 ml of dichloromethane, combine the dichloromethane layers, wash with 1.5 L of water, dry over anhydrous magnesium sulfate, concentrate to dryness to give a light yellow oil, pass through a silica gel column (purchased from Qi...
Embodiment 2
[0061] Preparation of 16,16-difluoro-13,14-dihydro-15-carbonyl-11-[(tetrahydro-2H-pyran-2-yl)oxy]-PGE 2 Benzyl ester 2
[0062]
[0063] At room temperature, 16,16-difluoro-13,14-dihydro-15(R,S)-hydroxy-11-[(tetrahydro-2H-pyran-2-yl)oxy]-PGF 2a Benzyl ester (54.3g) was dissolved in dichloromethane (340ml), N 2 Under protection, cool to 5°C, dissolve Dess-Martin oxidant (157.7g) in dichloromethane (1300ml) with stirring, drop into the above benzyl ester solution, and finish dropping in 20 minutes. Rise to 20°C and stir for 1h. TLC detects that the reaction is complete.
[0064] A 1.3 L aqueous solution of 377.6 g of sodium thiosulfate pentahydrate and 139.8 g of sodium bicarbonate was slowly added. Stir for 5 min, separate layers, back-extract the water layer with 200 ml of dichloromethane, combine the dichloromethane layers, wash with 1.5 L of water, dry over anhydrous magnesium sulfate, concentrate to dryness to give a light yellow oil, pass through a silica gel column...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com