Preparation method for clopidogrel intermediate
A technology for chlorine compounds and chlorophenylglycine, which is applied in the preparation of organic compounds, chemical instruments and methods, and cyanide reaction preparation, etc., can solve the problems of production cost and the increase of three wastes, avoid splitting and racemization, and solve the reaction The effect of difficult industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
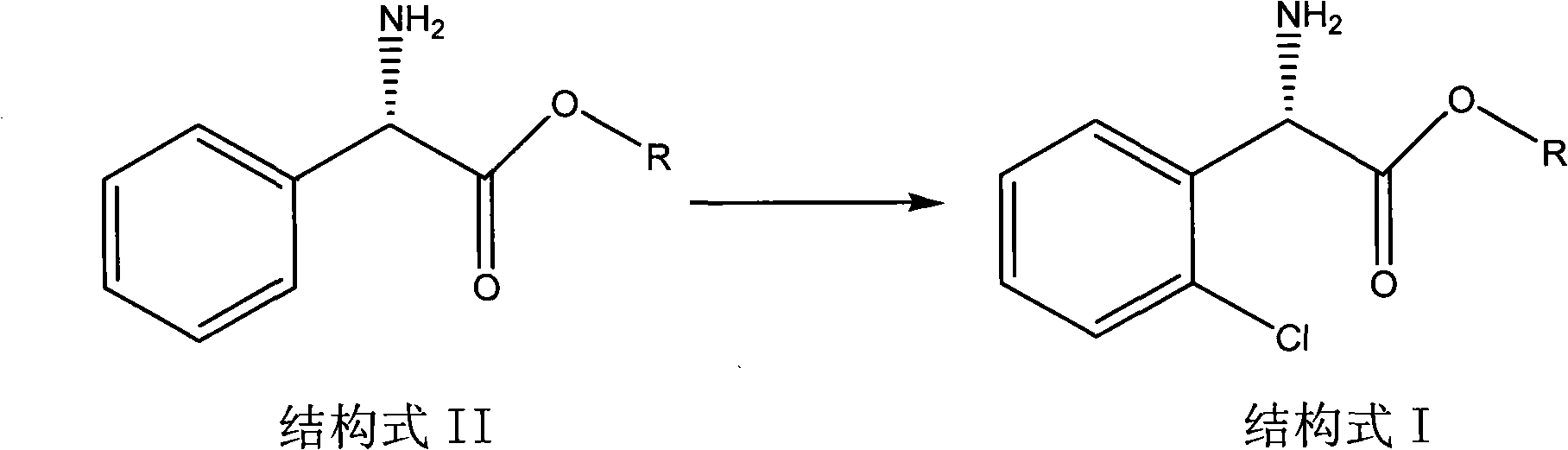
[0024] Embodiment 1: the preparation of (S)-2-chlorophenylglycine
[0025] Dissolve 5 grams of L-phenylglycine (33 mmol) in 15 milliliters of concentrated sulfuric acid, add 5.7 grams of N-chlorosuccinimide (36 mmol), heat up to 110 ° C for 5 hours, and detect no raw material spots by thin-plate chromatography 200 ml of water was added, and 5.6 g of the product was obtained by filtration, with a yield of 90%.
Embodiment 2
[0026] Embodiment 2: the preparation of (S)-2-chlorophenylglycine methyl ester hydrochloride
[0027] Dissolve 5 grams of L-phenylglycine methyl ester hydrochloride (23.6 mmol) in 15 milliliters of concentrated sulfuric acid, add 4.0 grams of N-chlorosuccinimide (26 mmol), heat up to 110 ° C for 5 hours, and the thin plate layer Add 200 milliliters of water after analysis and detection of no raw material spots, alkalinize with 2N sodium hydroxide, extract three times with dichloromethane, combine organic layers, dry, concentrate and treat with hydrogen chloride ethanol solution, filter to obtain 5.3 grams of product, yield 89% .
Embodiment 3
[0028] Embodiment 3: the preparation of (S)-2-chlorophenylglycine
[0029] Dissolve 5 grams of L-phenylglycine (33mmol) in 25 milliliters of chloroform, add 1.5 grams of ALCl3 (11mmol), heat up to reflux for 12 hours, no raw material spots are detected by thin plate chromatography, filter and refine to obtain 4.7 grams of product, yield 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com