Preparation and application of sulfonitride heavy metal ion chelating polymer microspheres
A technology of heavy metal ions and chelating polymers, which is applied in the preparation of microspheres, microcapsule preparations, water pollutants, etc., can solve the problems of slow release of chelating agents, decreased chelating ability, leakage pollution, etc., and achieves low production costs, Ease of operation, avoiding the effects of release and leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of sulfur nitrogen heavy metal ion chelating polymer microspheres: 2 mmol of polyvinyl alcohol was dissolved in 2 mol of deionized water to obtain an aqueous phase. Put 10mmol 4-vinylpyridine, 10mmol polyethylene glycol methacrylate, and 2.5mmol thiodiethylene glycol dimethacrylate into a sample bottle, add 70mmol n-octanol to dissolve, and then add benzoyl peroxide 0.058 g (0.24mmol), after complete dissolution, a dispersed phase was obtained. Mix the obtained aqueous phase and dispersed phase, replace with nitrogen for 10 minutes, remove oxygen, seal the tube, place in a constant temperature shaking water bath, keep at 82°C for 4 hours, then at 90°C for 2 hours. After the reaction is finished, cool to room temperature, and centrifuge at a speed above 15,000 rpm on a centrifuge to obtain a crude product of polymer microspheres. Re-suspend the microspheres in ethanol, then centrifuge, repeat the operation twice, and deionized water three times to remove the...
Embodiment 2-15
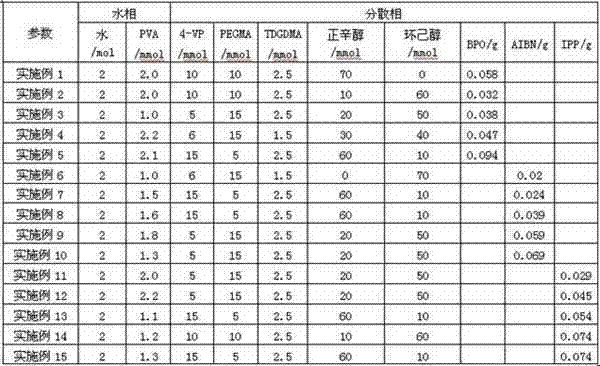
[0020] The operation process is the same as in Example 1, and the specific parameters of the aqueous phase and the dispersed phase are shown in Table 1.
[0021]
[0022] Table 1
[0023]
Embodiment 16
[0025] Application of heavy metal chelating polymer microspheres: The circuit board wastewater from a company in Suzhou was selected, and the measured copper ion concentration was 128 mg / L, and the pH was 5.2. Add 2g·L -1The heavy metal ion chelating polymer microspheres of Examples 1 to 15 were stirred at room temperature for 3 minutes at a stirring rate of 250 rpm, then left to filter, and the supernatant was taken to measure the copper ion concentrations of 0.0597mg / L and 0.0691mg / L respectively. L, 0.0582mg / L, 0.0787mg / L, 0.130mg / L, 0.423mg / L, 0.310mg / L, 0.284mg / L, 0.261mg / L, 0.356mg / L, 0.0645mg / L, 0.0723mg / L L, 0.0816mg / L, 0.0633mg / L, 0.0836mg / L, the concentration of copper ions all meet the national emission standards.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com