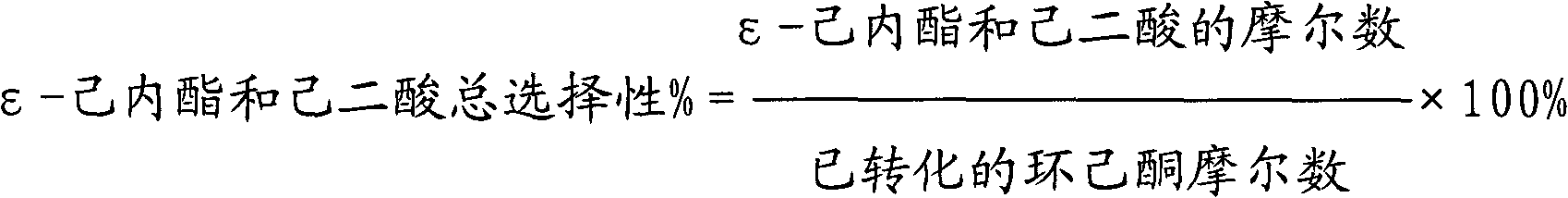Method for preparing caprolactone and adipic acid
A technology of adipic acid and caprolactone, applied in chemical instruments and methods, preparation of carboxylate, preparation of organic compounds, etc., can solve problems such as harmful emissions, achieve simple production process, overcome complex production process, and be environmentally friendly Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The mol ratio of cyclohexanone, hydrogen peroxide, solvent and catalyst (zinc nitrate and titanium silicon molecular sieve 0.2) is 1: 2 according to the mol ratio of cyclohexanone and hydrogen peroxide, and the mass ratio of solvent acetonitrile and catalyst is 20, The mass ratio of cyclohexanone to the catalyst is 100, and the reaction is carried out at a temperature of 90° C. and a pressure of 1.0 MPa.
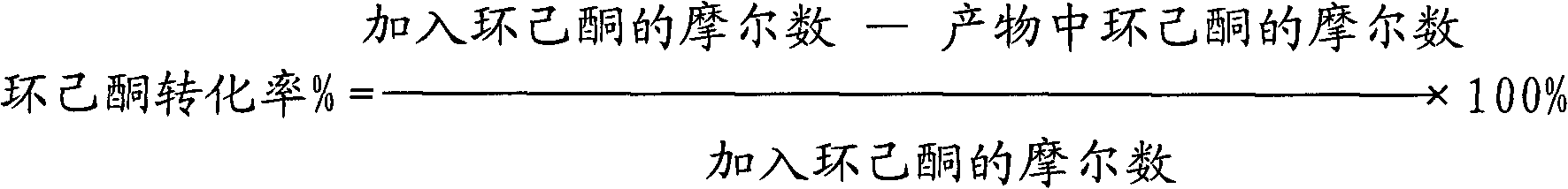
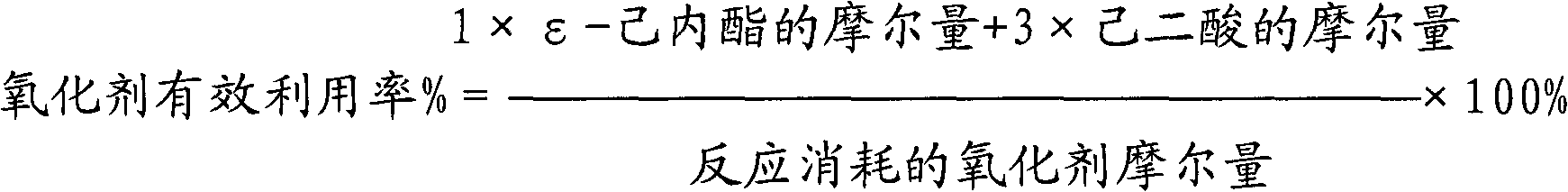
[0044] The results of the 2-hour reaction were as follows: the conversion rate of cyclohexanone was 86%; the effective utilization rate of hydrogen peroxide was 72%; the total selectivity of ε-caprolactone and adipic acid was 91%.
[0045] The results of the 12-hour reaction were as follows: the conversion rate of cyclohexanone was 75%; the effective utilization rate of hydrogen peroxide was 71%; the total selectivity of ε-caprolactone and adipic acid was 90%.
Embodiment 2
[0047] The mol ratio of cyclohexanone, hydrogen peroxide, solvent and catalyst (zinc phosphate and titanium silicon molecular sieve 1) is 1: 10 according to the mol ratio of cyclohexanone and hydrogen peroxide, and the mass ratio of solvent acetone and catalyst is 50, The mass ratio of cyclohexanone to the catalyst is 1, and the reaction is carried out at a temperature of 60° C. and a pressure of 2.5 MPa.
[0048] The results of the 2-hour reaction were as follows: the conversion rate of cyclohexanone was 89%; the effective utilization rate of hydrogen peroxide was 75%; the total selectivity of ε-caprolactone and adipic acid was 93%.
[0049] The results of the 12-hour reaction were as follows: the conversion rate of cyclohexanone was 87%; the effective utilization rate of hydrogen peroxide was 73%; the total selectivity of ε-caprolactone and adipic acid was 91%.
Embodiment 3
[0051] The mol ratio of cyclohexanone, hydrogen peroxide, solvent and catalyst (zinc sulfate and titanium silicon molecular sieve 0.1) is 1: 3 according to the mol ratio of cyclohexanone and hydrogen peroxide, and the mass ratio of solvent propionitrile and catalyst is 150 , the mass ratio of cyclohexanone to the catalyst is 2, and the reaction is carried out at a temperature of 50° C. and a pressure of 0.5 MPa.
[0052] The results of the reaction for 2 hours are as follows: the conversion rate of cyclohexanone is 81%; the effective utilization rate of hydrogen peroxide is 73%; the total selectivity of ε-caprolactone and adipic acid is 91%.
[0053] The results of the 12-hour reaction were as follows: the conversion rate of cyclohexanone was 67%; the effective utilization rate of hydrogen peroxide was 66%; the total selectivity of ε-caprolactone and adipic acid was 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com