Method for preparing N-(3,5-dichloropyridyl-4-yl)-3-cyclopropylmethoxy-4-difluoromethoxybenzoyl amine
A technology of difluoromethoxybenzamide and cyclopropylmethoxy, which is applied in the field of medicinal chemistry preparation, can solve the problems of difficult impurity control, optimization of synthesis method, low yield and the like, and achieves simple and convenient post-processing. Production operations, effects of high purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
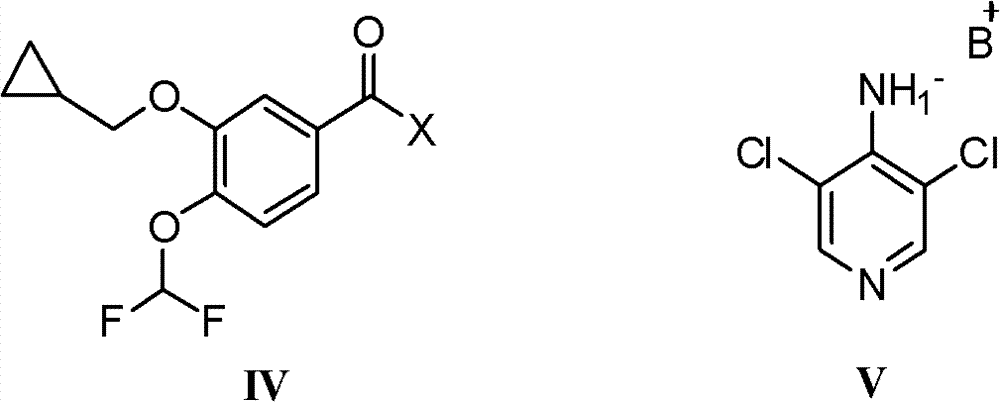
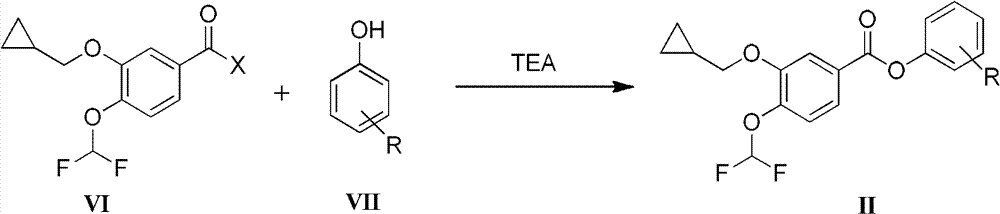
[0034] Embodiment 1: Preparation of 3-cyclopropylmethoxy-4-dichloromethoxybenzoic acid p-nitrophenol
[0035]
[0036] Under nitrogen protection and stirring in an ice bath, 3.31g of p-nitrophenol was added to 60ml of dichloromethane, and partly dissolved to form a suspension. After adding 6.6ml of triethylamine, p-nitrophenol was completely dissolved, and 5.66g of 3- A solution of cyclopropylmethoxy-4-difluoromethoxybenzoic acid freshly prepared acid chloride in 30 ml of dichloromethane was added dropwise to the above solution, and the temperature was raised to 20° C. and stirred after the dropwise addition. TLC showed that after the completion of the reaction, the reaction solution was concentrated under reduced pressure, and 50ml of absolute ethanol was added to the residue to stir, filtered, washed with 50ml of water, and washed with a small amount of absolute ethanol to obtain a white solid, which was dried to obtain 7.65g of the product, with a content > 98%, two steps...
Embodiment 2
[0037] Embodiment 2: the preparation of roflumilast
[0038]
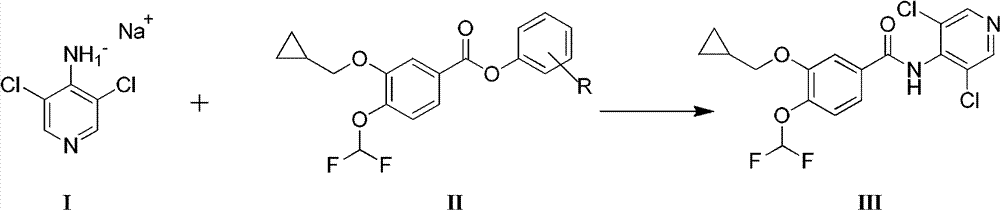
[0039] Under nitrogen protection and stirring in an ice bath, 3.24g of 4-amino-3,5-dichloropyridine was dissolved in 30ml of N,N-dimethylformamide, and 1.44g of sodium hydride (60%) was added in batches, and kept stirring After 15 minutes, a solution of 6.84g of 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid p-nitrophenyl in 45ml of N,N-dimethylformamide was added dropwise to the above mixture After the dropwise addition, the temperature was raised to 10°C and stirred. About 15 minutes later, TLC showed that the reaction was complete. Slowly add 10ml of water dropwise under ice bath, pour the reaction liquid into 350ml of water, adjust the pH to 3.2, a large amount of white solid precipitated, filtered, washed with a small amount of water and dried in vacuum to obtain the crude product of roflumilast 6.89 g.
[0040] Add 45ml of 90% (V / V) ethanol to the above-mentioned roflumilast crude product, heat and r...
example 3
[0042] Example 3: Preparation of Roflumilast
[0043]
[0044] Under nitrogen protection and stirring at 20°C, 1.75g of 4-amino-3,5-dichloropyridine was dissolved in 15ml of N,N-dimethylformamide, 0.87g of sodium hydroxide was added, and the 2.74 The solution of g 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid p-nitrophenyl ester in 20ml N,N-dimethylformamide was added dropwise to the above mixture, and the stirring was continued at 50°C After 5 hours, TLC showed that the reaction was complete. The reaction solution was poured into 180ml of water, and the pH was adjusted to 2. A large amount of white solid precipitated, filtered, washed with a small amount of water, and dried in vacuum to obtain 2.12g of crude roflumilast.
[0045] Add 15ml of 80% (V / V) ethanol to the above-mentioned roflumilast crude product, heat and reflux for 50 minutes, stir, and after clarification, cool and crystallize naturally for 8 hours, separate out the solid filter, and obtain refined rof...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com