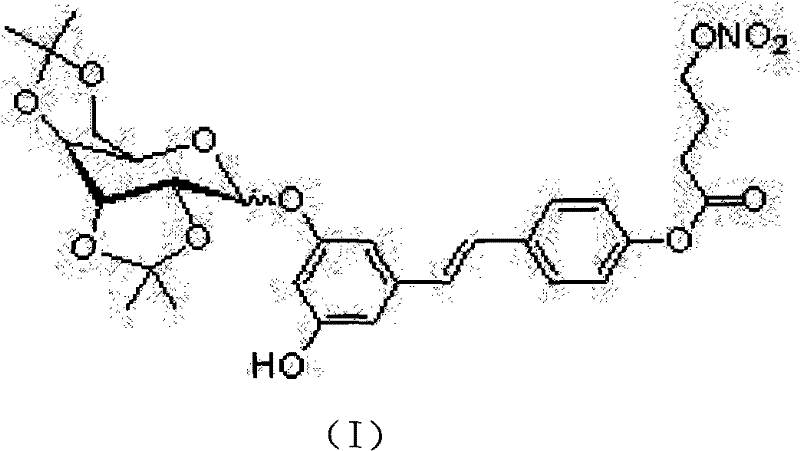
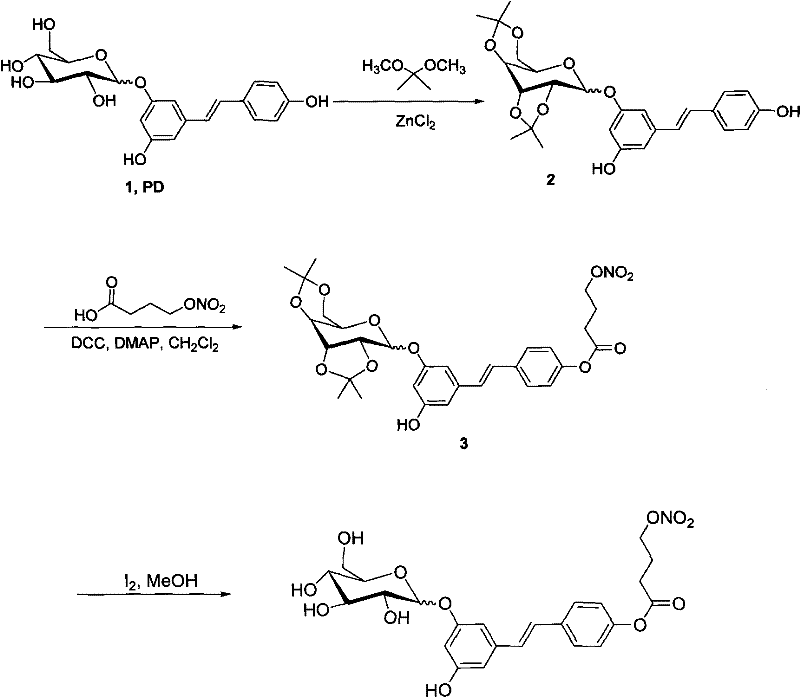
NO (nitric oxide) donor type polydatin (PD) derivative as well as preparation method and medical application thereof
A technology of nitric oxide and polygoside, which is used in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The pharmacological experiment and the result of compound of the present invention are as follows:
[0022] 1. In vitro experiment, determination of nitric oxide release
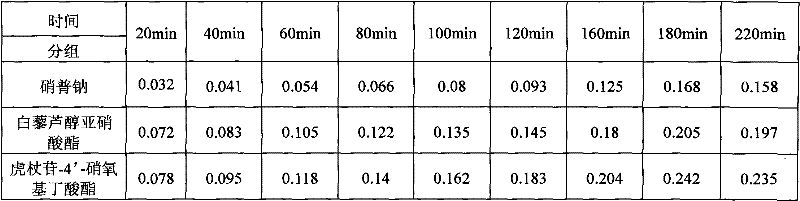
[0023]The polydatin-4'-nitroxybutyrate compound was made into 2.5mmol / L DMSO solution, and 1ml was mixed with 3.4mmol / L L-cysteine or no L-cysteine respectively. The PBS solution was diluted to 100ml, and the final concentration of the tested compound was 0.025mmol / L. After incubating the solution at 37°C, 8ml and 2ml of Griess reagent [4g p-sulfanilamide, 0.2g N-(1-naphthyl)ethylenediamine dihydrochloride, 10mL mass fraction were taken out at each time point. 85% phosphoric acid, dilute to 100ml with distilled water], mix at room temperature for 10min, and measure the absorbance at 540nm. The results are shown in Table 1.
[0024] Table 1 Comparison of NO release from polydatin-4'-nitroxybutyrate
[0025]
[0026] The results showed that the release amount of the target compound polydatin-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com