Preparation method of optically active phenyl glycidol and derivatives thereof
A phenylglycidol, optically active technology, applied in the field of biochemistry, can solve problems such as unfavorable production process, waste of resources, cumbersome splitting steps, etc., to improve utilization rate, improve utilization, avoid metal catalysts and organic reagents pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~7E
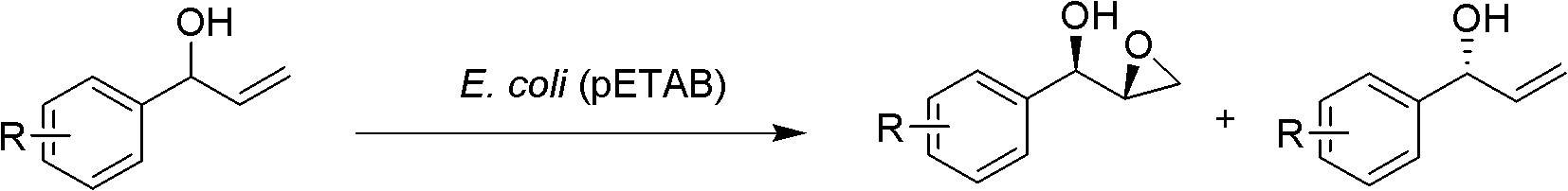
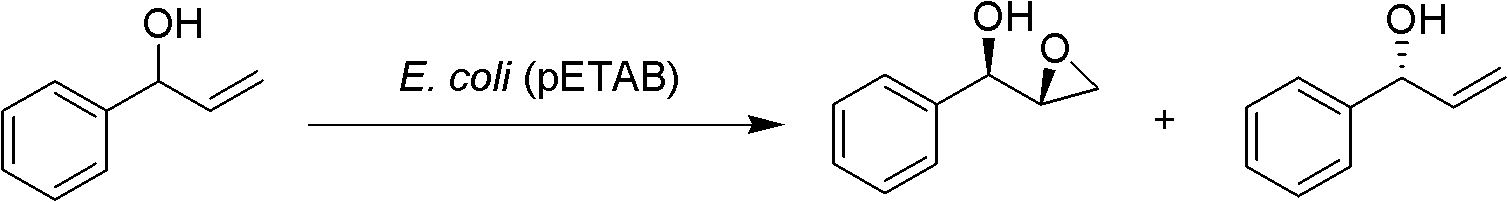
[0015] Embodiment 1~7E.coli (pETAB) selectively catalyzes (±)-1-phenyl-2-propenol epoxidation
[0016]
Embodiment 1
[0018] The expression strain E.coli (pETAB) constructed according to the method described in the patent "A Styrene Cyclooxygenase Gene and Its Use" is preserved on the LB plate (kanamycin resistance), and the E.coli (pETAB) is picked ) single bacterium colony, at 37 ℃ in the LB medium containing kanamycin (501 μ g / ml), 230rpm culture 16h as seed culture medium, inoculate in TB medium with 1% inoculum size, 37 ℃ shaking culture 3h, Then lower the temperature to 20°C to induce the expression of the styAB gene, and after 18-21 hours of induction, refrigerate and centrifuge at 4°C to harvest the bacteria.
[0019] Take 2 g of freshly cultured wet cells of E. coli (pETAB), suspend in 20 mL of potassium phosphate buffer (0.1 M, pH 7.0), and add 12 mg of (±)-1-phenyl-2-propenol. The reaction was shaken on a shaker (30° C., 230 rpm) for 2 hours. After the reaction was completed, the reaction liquid was centrifuged (8500 rpm, 5 min, 4° C.). The supernatant was extracted with ether (2×...
Embodiment 2
[0026]The reaction temperature is 20°C, the substrate is 20 mg, and the conversion takes 24 hours. Other operations are as in Example 1, and all 10 mg of (S)-1-phenyl-2-propenol can be converted into (1R,2R)-1-phenyl- 2,3-Glycidyl alcohol, and residual (R)-1-phenyl-2-propenol.
[0027] The product data is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com