Primer sequences and quantitative determination kit used for simultaneously detecting human CMV and BK virus DNA
A quantitative detection and virus technology, applied in the direction of recombinant DNA technology, DNA / RNA fragments, microbial measurement / inspection, etc., to achieve the effect of multi-experimental information, simple composition, and reduced manpower and material resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 2
Embodiment 2
[0033] Example 3
Embodiment 3
[0035] Reagent A: A 250 μL solution is composed of a mixture of primers and probes for CMV and BKV. The solution contains 5 μM SEQ ID NO.1, 5 μM SEQ ID NO.2, 5 μM SEQ ID NO.4, 5 μM SEQ ID NO.5 and 2 μM probe SEQ ID NO.3, 2 μM probe SEQ ID NO.6;
[0036] Reagent B: Consists of 250 μL buffer containing 1 mM dNTPs (including dATP, dCTP, dGTP, dTTP), 200 mM Tris-HCl, 200 mM KCl, 100 mM (NH 4 ) 2 SO 4 , 45 mM MgSO 4 ;
[0037] Reagent C: 5U / μL Taq DNA polymerase;
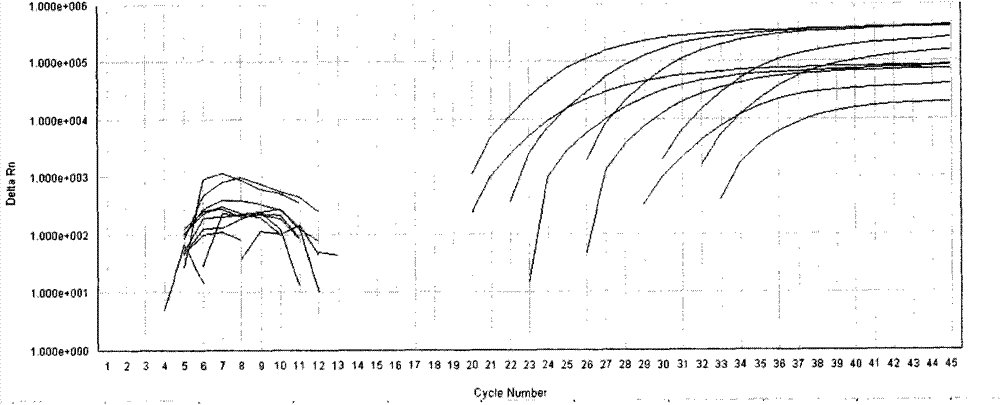
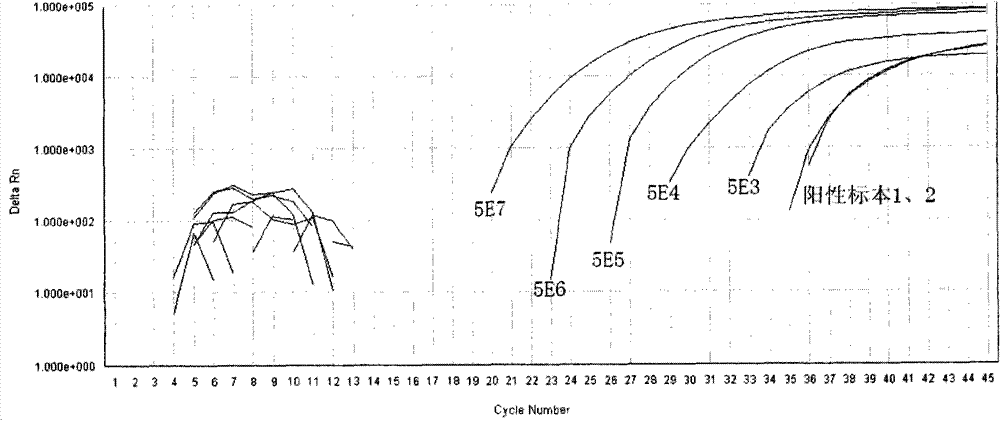
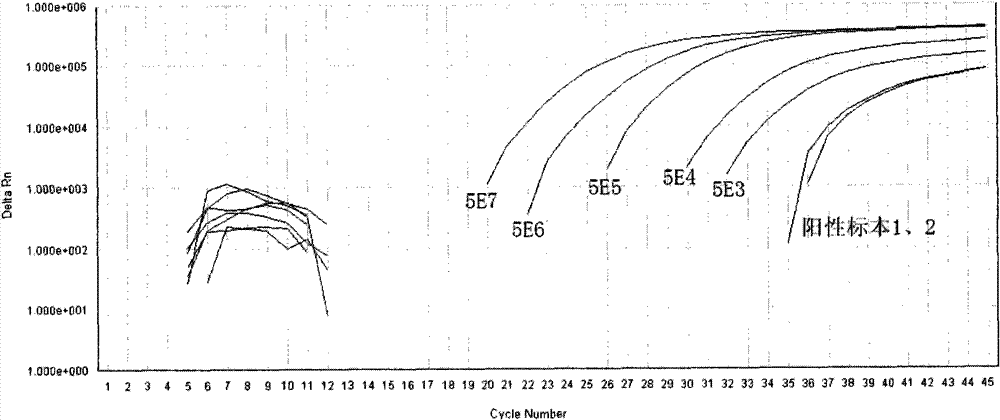
[0038] Reagent D: The prepared standard was serially diluted to reach 5E7 (5×10 7 copy / mL), 5E6, 5E5, 5E4, 5E3, used for standard curve formulation;
[0039] Reagent E: negative control (normal human DNA without CMV and BKV infection);
[0040] Reagent G: Positive control (using 5E4 standard).
[0041] Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com