Method for preparing 2,3,3,3-tetrafluoropropene
A technology of tetrafluoropropene and tetrachloropropene, which is applied in 2 fields, can solve the problem that the reaction cannot be completed in one step in a single reactor, and achieves the effects of mild reaction conditions, simple process, and energy and resource saving.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
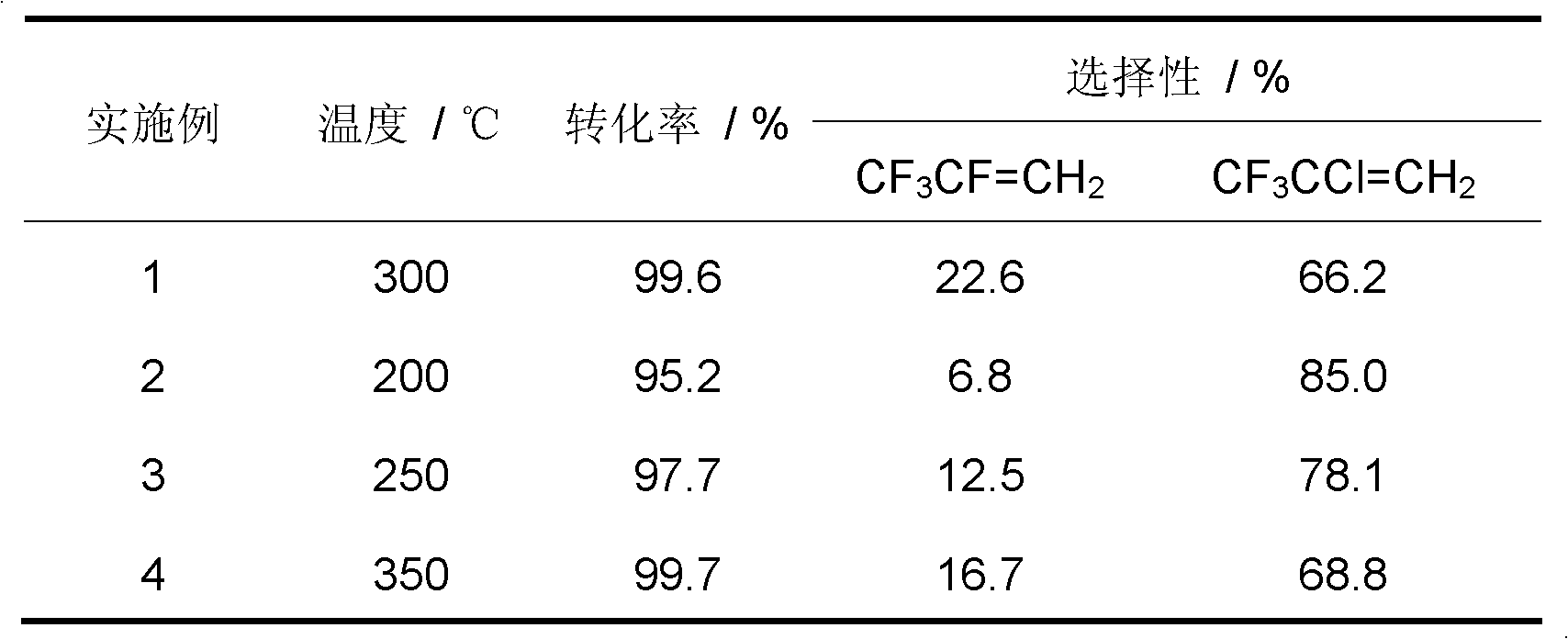
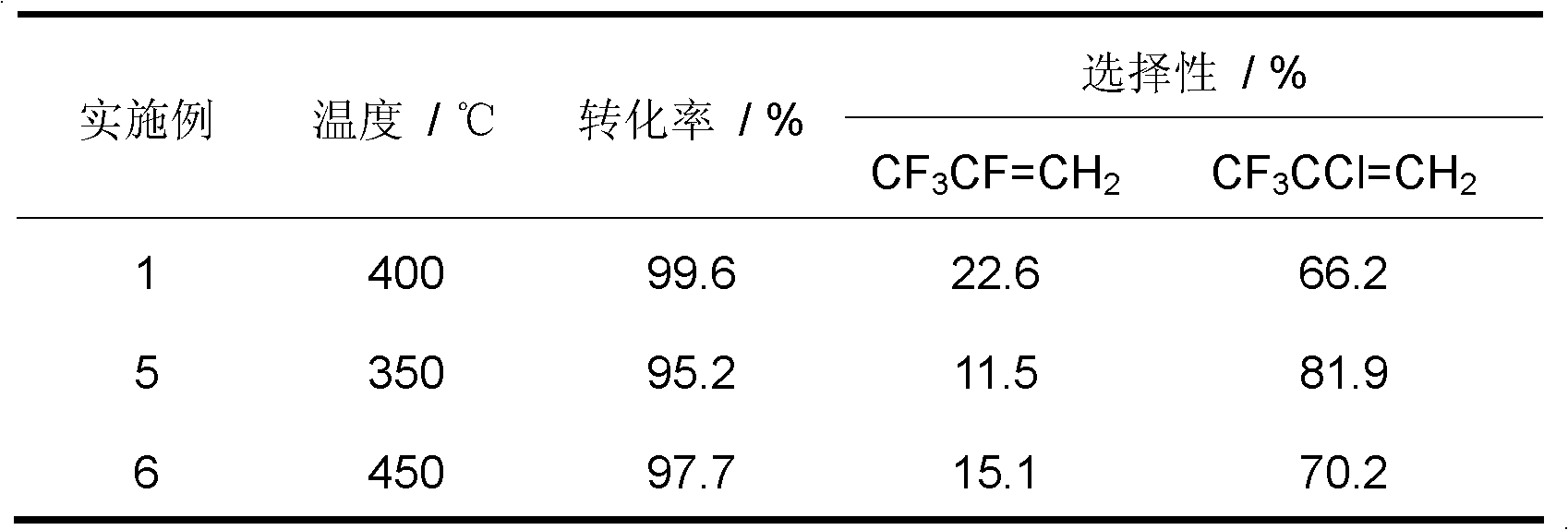
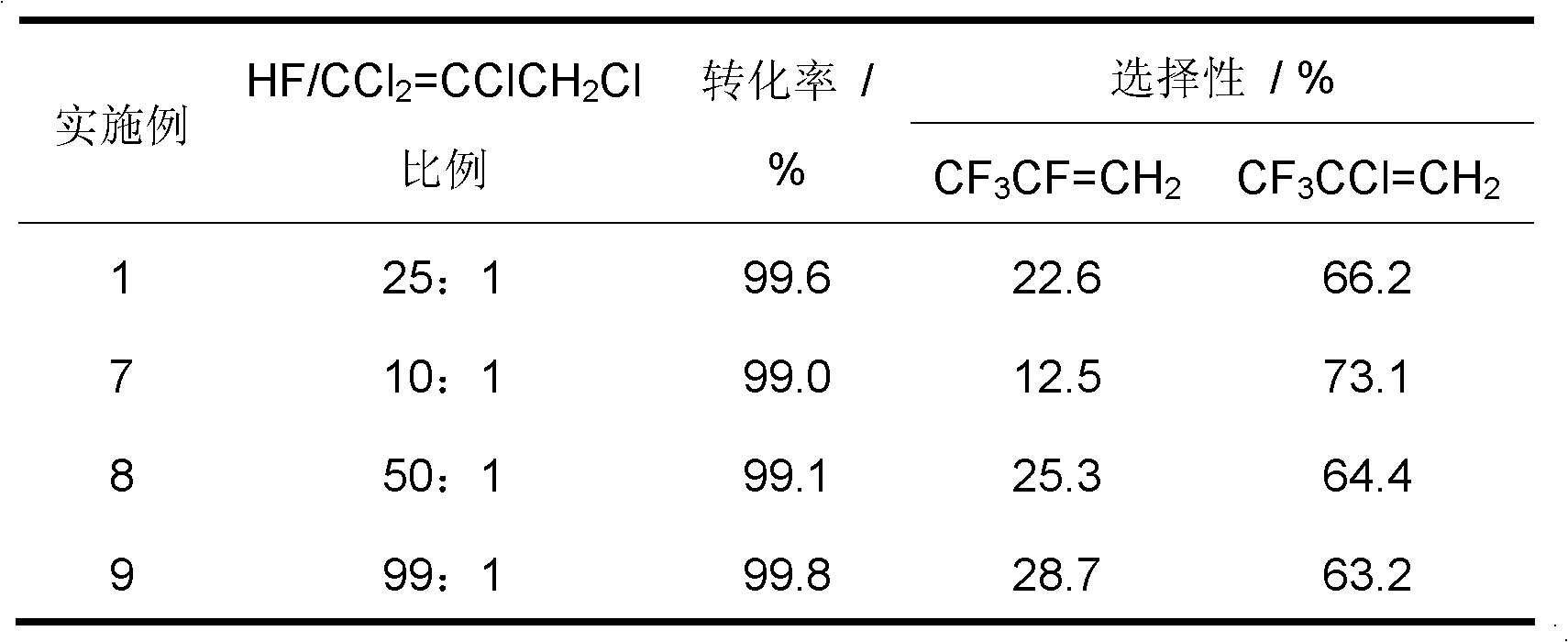
[0034] Weigh a certain amount of Cr (NO 3 ) 3 ·9H 2 O, add water to Cr(NO 3 ) 3 ·9H 2 After O is dissolved, use (NH 4 ) 2 CO 3 Adjust the pH of the solution to 7-8, so that the dissolved chromium ions are converted into hydroxide precipitates, then centrifuged, suction filtered, and then the precipitates were dried at 120 °C overnight, and the dried substances were dried at 500 °C, N 2 calcined for 4h in the atmosphere to obtain Cr 2 O 3 The catalyst, after tableting, is charged into the reactor.
[0035] inner diameter In the nickel alloy single reactor, add 6ml of the above Cr 2 O 3 The catalyst was dewatered under a nitrogen atmosphere, and then fluorinated with hydrogen fluoride gas. The catalyst was equally divided into upper and lower sections, which were controlled by their respective reaction temperatures. The temperature of the catalyst in the upper section of the reactor was 300 °C, and the temperature of the catalyst in the lower section of the reactor...
Embodiment 2
[0037] The same operation as in Example 1 was performed, except that the reaction temperature of the catalyst in the upper stage of the reactor was changed to 200° C. The reaction results are shown in Table 1.
Embodiment 3
[0039] The same operation as in Example 1, the difference is that the reaction temperature of the catalyst in the upper stage of the reactor is changed to 250° C. The reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com