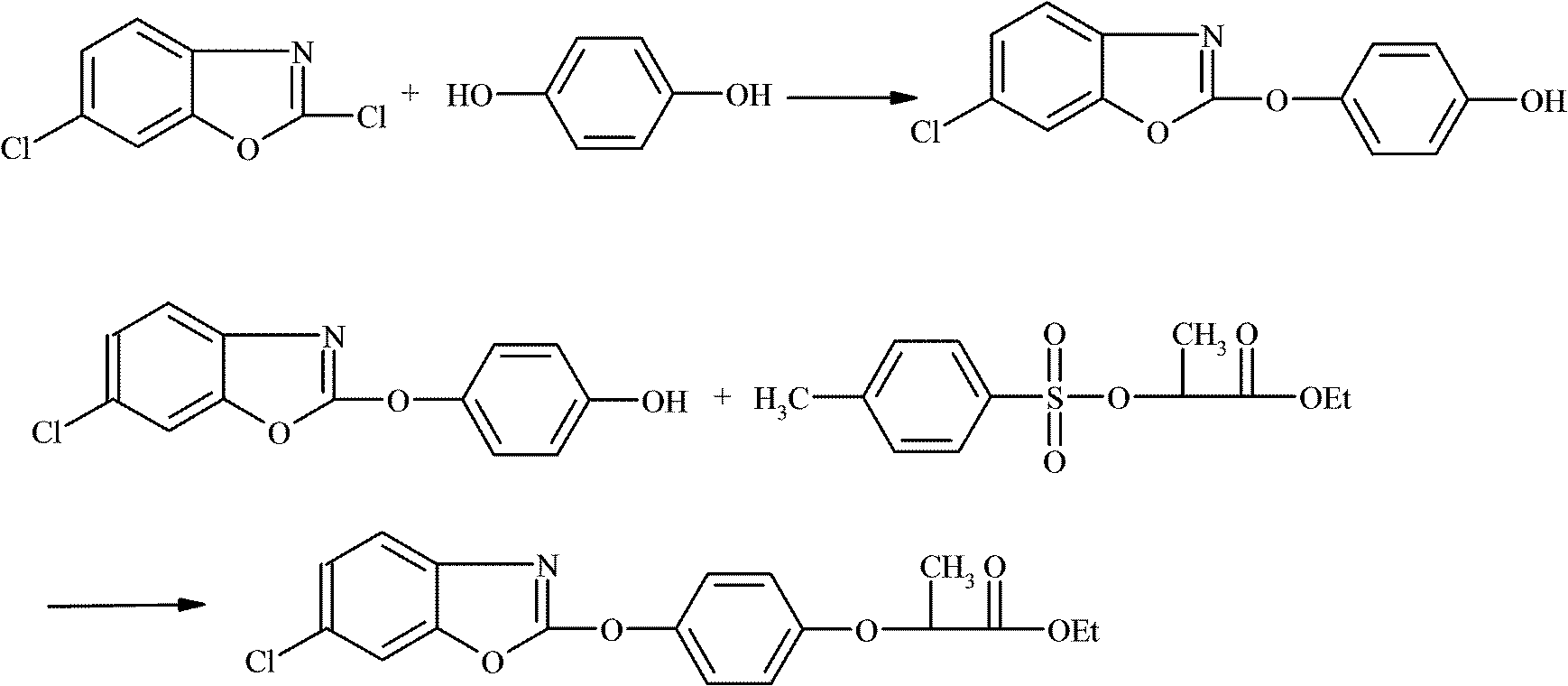
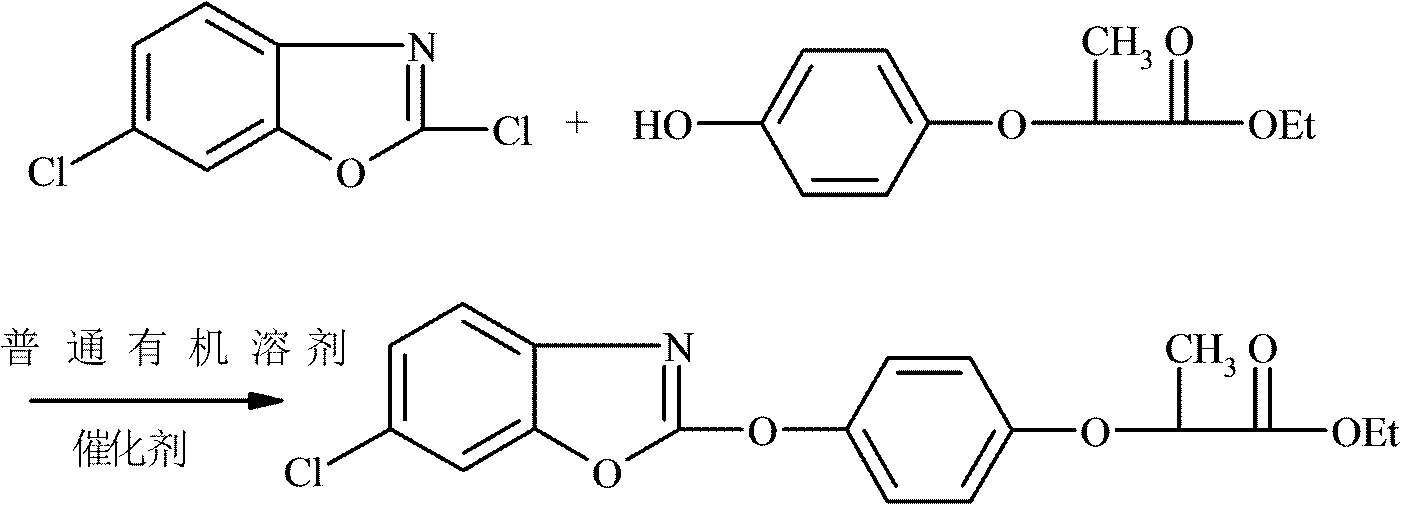
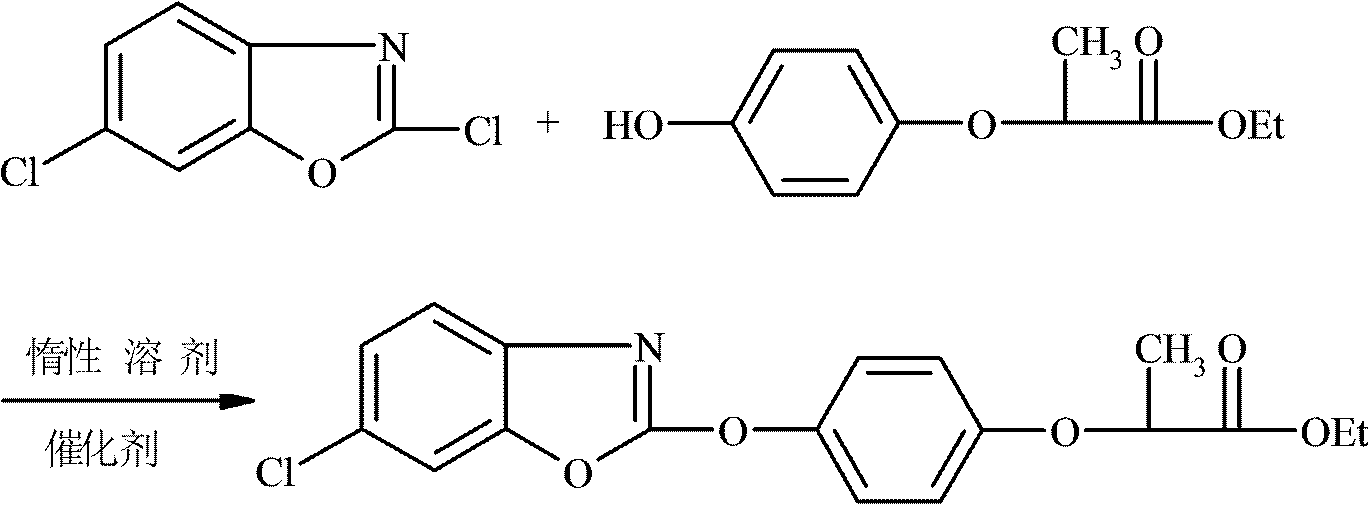
New method for synthesizing fenoxaprop-P-ethyl
A technology for the synthesis of fenoxaprop-ethyl and its synthesis method, which is applied in the field of herbicide synthesis, can solve the problems of many by-products, large pollution, and low yield, and achieve products with high optical activity, high optical content, and high chemical content. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 500ml four-necked flask, drop into 200ml toluene, 21gR-(+)-2-(4-hydroxyphenoxy) ethyl propionate (0.1mol), 1.05g polyethylene glycol 600 and 17.4g anhydrous potassium carbonate ( 0.125mol), start stirring and mix at a stirring speed of 200rmp, heat up to 50°C while stirring until completely dissolved, add 19.7g of 2,6-dichlorobenzoxazole (0.105mol) dropwise at 60-65°C After about 2 hours of dripping, after the dripping, keep warm at 60-65°C for 6 hours, cool to room temperature and filter after the reaction, wash the filtrate with 2*50g water, let stand to separate the lower water layer, and distill the organic layer under reduced pressure The toluene was directly recovered and used mechanically, and the crude product was precipitated, and recrystallized with ethanol to obtain 35.46 g of refined oxaprop-ethyl white needle-like crystals, with a melting point of 80-84 ° C, a product purity of 98.95%, an effective optical body content of 99.7%, and a molar yield of the...
Embodiment 2
[0027] Drop into 200ml toluene, 21gR-(+)-2-(4-hydroxyphenoxy) ethyl propionate (0.1mol), 1.05 gram polyethylene glycol 6000 and 17.4g anhydrous potassium carbonate ( 0.125mol), start stirring and mix at a stirring speed of 200rmp, heat up to 50°C while stirring until completely dissolved, add 19.7g of 2,6-dichlorobenzoxazole (0.105mol) dropwise at 60-65°C , about 2 hours to drop, after the drop, keep warm at 60-65°C for 6 hours, cool to room temperature and filter after the reaction, wash the filtrate with 2*50g water, stand at 50°C to separate the lower water layer, and depressurize the organic layer The toluene was distilled and directly recovered and used mechanically. The crude product was precipitated and recrystallized with ethanol to obtain 35.27 g of white needle-like crystals of fenoxaprop-ethyl, with a melting point of 80-84 ° C, a product purity of 99.1%, and an effective optical body content of 99.5%. The product The molar yield is 96.58% (based on R-(+)-2-(4-hydro...
Embodiment 3
[0029] In a 500ml four-necked flask, drop 200ml of dichloroethane, 21g of R-(+)-2-(4-hydroxyphenoxy) ethyl propionate (0.1mol), 1.05 grams of polyethylene glycol 6000 and 17.4g of anhydrous Potassium carbonate (0.125mol), start stirring and mix at a stirring speed of 200rmp, heat up to 50°C while stirring until it dissolves completely, add 19.7g of 2,6-dichlorobenzoxazole ( 0.105mol), drop it in about 2 hours, keep it warm at 60-65°C for 6 hours after the drop, cool to room temperature and filter after the reaction, wash the filtrate with 2*50g water, let it stand to separate the lower water layer, and decompress the organic layer Ethylene dichloride was distilled and directly recovered and used mechanically. The crude product was precipitated and recrystallized with ethanol to obtain 35.3 g of refined fenoxaprop-ethyl white needle crystals with a melting point of 80-84°C. The product had a purity of 99.5% and an effective optical body content of 99% %, the product molar yield...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com