Soluble polyfunctional (meth)acrylate copolymer, method for producing same, curable resin composition, and cured product
An acrylate and acrylate-based technology, applied in the direction of instruments, optics, optical components, etc., can solve the problems of resin composition strength, heat resistance reduction, and insufficient heat resistance, and achieve improved adhesion and heat resistance retention Performance improvement and excellent optical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
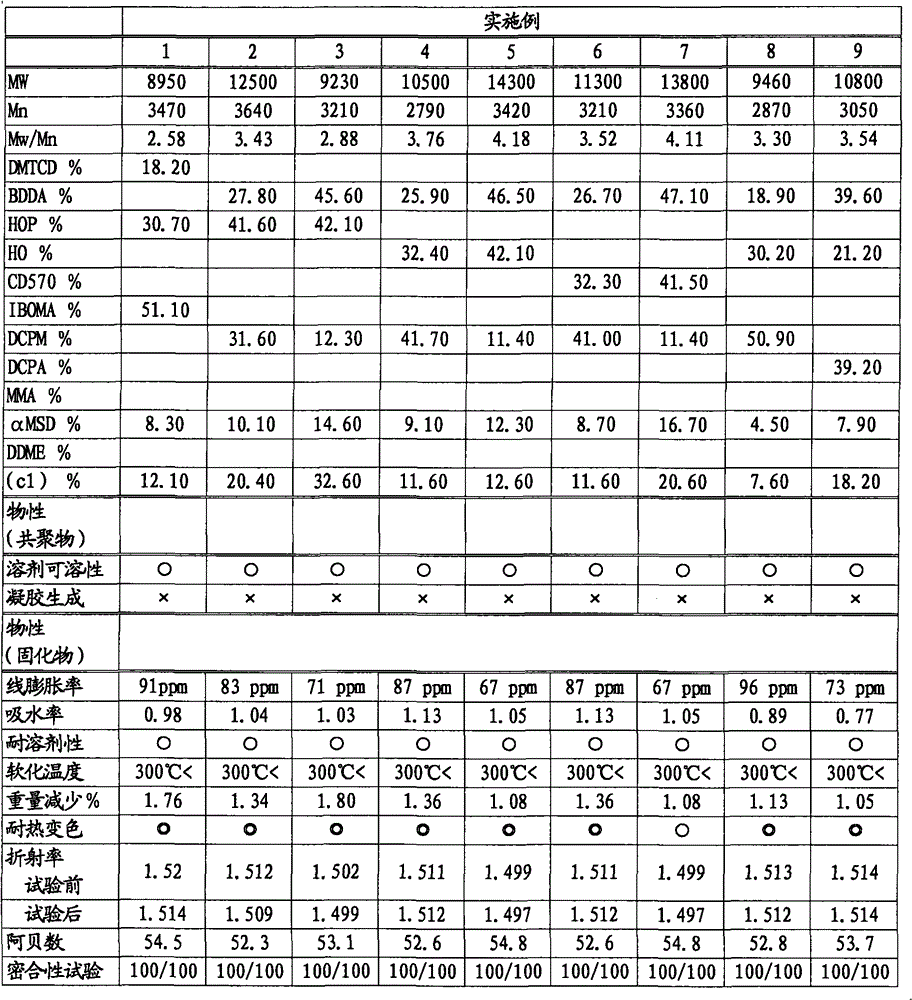
[0168] Put 3.2 mol (926.5 ml) of dimethylol tricyclodecane diacrylate, 8.0 mol (1814.1 ml) of isobornyl methacrylate, and 4.8 hydroxypropyl methacrylate into a 10.0L reactor. mol (645.5ml), 1,4.8mol (1145.9ml) of 2,4-diphenyl-4-methyl-1-pentene, 2400ml of toluene, add 240mmol of benzoyl peroxide at 90°C to react 6 hour. After the polymerization reaction was stopped by cooling, the reaction mixture was poured into a large amount of hexane at room temperature to precipitate the polymer. The obtained polymer was washed with hexane, filtered, dried, and weighed to obtain 1392.6 g of copolymer A (yield: 40.5 wt%).
[0169] The Mw of the obtained copolymer A was 8950, Mn was 3470, and Mw / Mn was 2.58. Go through 13 C-NMR, 1 According to H-NMR analysis and elemental analysis, copolymer A contains a total of 18.2 mol% of structural units derived from dimethylol tricyclodecane diacrylate, a total of 51.1 mol% of structural units derived from isobornyl methacrylate, and 30.7 mol% A struc...
Embodiment 2~13 and comparative example 1~6
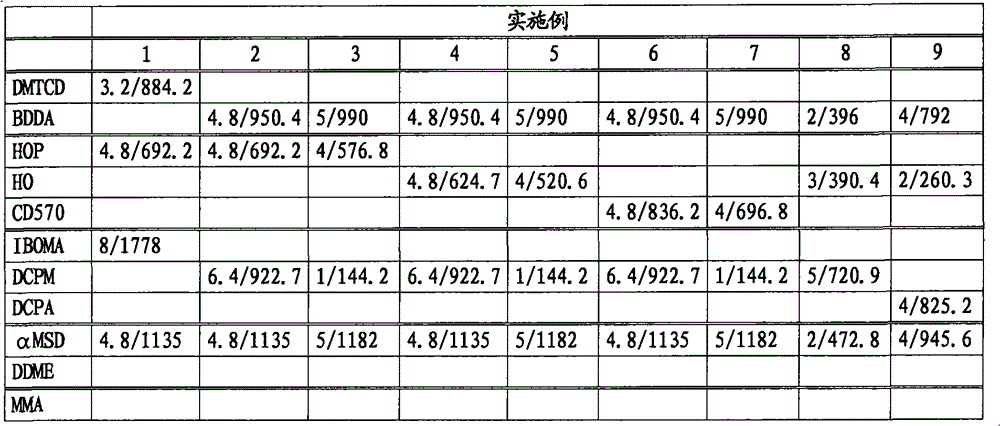
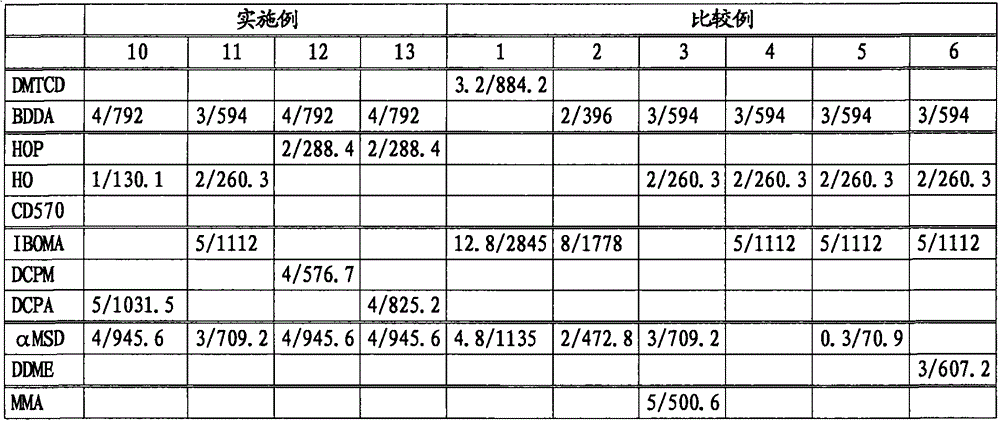
[0177] Using various bifunctional acrylates and monofunctional (meth)acrylates, polymerization was carried out in the same manner as in Example 1 with the raw material composition shown in Table 1.
[0178] Tables 1 and 2 show the amount of raw materials used in the reaction, and Tables 3 and 4 show the test results of the copolymer and its cured product. Unless otherwise specified, other reaction conditions and measurement conditions are the same as in Example 1. In Table 1, the amount of raw materials used is expressed in mol and weight (g), and the stated format is mol / g.
[0179] The ellipsis used in the table is shown below.
[0180] DMTCD: Dimethylol tricyclodecane diacrylate (c)
[0181] BDDA: 1,4-butanediol diacrylate (c)
[0182] HOP: 2-hydroxypropyl methacrylate (b)
[0183] HO: 2-hydroxyethyl methacrylate (b)
[0184] CD570: Partially ethoxylated 2-hydroxyethyl methacrylate (b)
[0185] IBOMA: Isobornyl methacrylate (a)
[0186] DCPM: tricyclo[5.2.1.02,6]dec-8-yl methacrylate (...
Synthetic example 1
[0215] In the same manner as in Example 1, copolymer A was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com