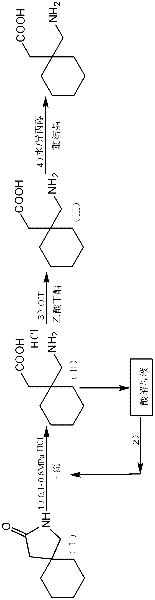
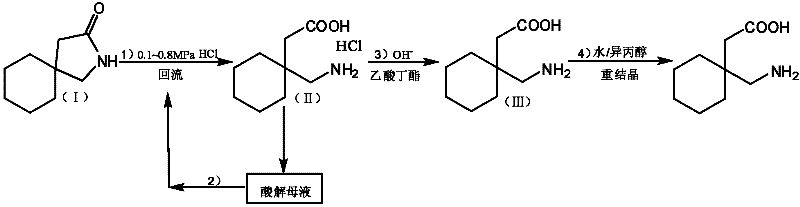
Method for preparing high-purity gabapentin
A technology of gabapentin and high purity, applied in the field of preparation of high-purity gabapentin, can solve the problems of affecting the purity of finished products, large amount of strong base, insufficient purity, etc., and achieves improvement of purity and yield, total yield, and reaction yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: a kind of preparation method of high-purity gabapentin, carries out following steps successively:
[0054] 1), the preparation of gabapentin hydrochloride:
[0055] In a 500ml autoclave, add 2-aza-spiro[4,5]-3-decanone 40g, water 40g, refined hydrochloric acid 100g (refined hydrochloric acid is the hydrochloric acid aqueous solution that mass concentration is 36%), seals reaction system, adds Press down to 0.8MPa, heat up to reflux. After reacting for 3 hours, it was slowly lowered to room temperature (cooling treatment with tap water), and the ice-water bath continued to cool down to 0-4 ° C, stirred at 0-4 ° C for 3 h, and filtered with suction to obtain 59.9 g of gabapentin hydrochloride wet product (measured moisture content 211 %, yield 87.2%), 45 ℃ oven dry weight 49.8g, purity 98.4% (HPLC); Obtain acidolysis mother liquor 109.0g, this acidolysis mother liquor is through titration (i.e. adopt titration method to measure the mass content of hydrochlo...
Embodiment 2
[0076] Embodiment 2, a kind of preparation method of high-purity gabapentin, carries out following steps successively:
[0077] 1), the preparation of gabapentin hydrochloride
[0078] Add 40 g of 2-aza-spiro[4,5]-3-decanone and 140 g of refined hydrochloric acid into a 500 ml autoclave, close the reaction system, pressurize to 0.5 MPa, and raise the temperature to reflux. After reacting for 4 hours, it was slowly lowered to room temperature, continued to cool down to 0-4°C in an ice-water bath, stirred at 0-4°C for 3 hours, and filtered with suction to obtain 55.8 g of gabapentin hydrochloride wet product (moisture content: 19.1%, yield: 83.3%) , dried at 45°C, weighing 47.8g, purity 98.0% (HPLC); 117.1g of acidolysis mother liquor was obtained, and the titration content of hydrochloric acid in the acidolysis mother liquor was 33.5%.
[0079] 2), the application of acidolysis mother liquor (1):
[0080] Add 40g of 2-aza-spiro[4,5]-3-decanone to a 500ml autoclave, 117.1g of ...
Embodiment 3
[0093] Embodiment 3, a kind of preparation method of high-purity gabapentin, the pressure in step 1), step 2) and step 3) is changed into 1.5MPa; All the other are the same as embodiment 1.
[0094] Use 2-aza-spiro[4,5]-3-decanone 120g in total, refined hydrochloric acid 165.2g, can get gabapentin crude product 127.8g (measure moisture content 9.3%), finally get gabapentin fine product 105.0g (total yield 78.3%) %, purity 99.9%), individual impurity is less than 0.05%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com