Preparation method of cobamamide lyophilized preparation for injection
A technology for adenosylcobalamin and freeze-dried preparations, which is applied in the field of preparation of adenosylcobalamin freeze-dried preparations for injection, and can solve problems such as stabilizer human health hazards, poor control of light-proof conditions, and unsatisfactory product stability. , to improve the stability of the
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Under a 50 lux red light, weigh an appropriate amount of adenosylcobalamin 1000mg and mannitol 30000mg, add it to water filled with nitrogen, stir to dissolve it, add 0.1% (g / ml) activated carbon, stir, take a sample, and detect the intermediate , coarse filtration, fine filtration, and packed in brown vials (adenosylcobalamin 10mg / support). Nitrogen protection. Arrange them in a freeze-drying box and freeze-dry them. After completion, fill the freeze-drying bottles with nitrogen gas, stopper them and take them out of the box.
[0015] Freeze-drying process:
[0016] Put the medicine into the front box of the freeze dryer, close the box door, and start to cool the front box. When the temperature of the product drops to -40°C, keep it warm for 2 hours, and then start to cool the back box. When the temperature of the back box is cooled to below -45°C, Start vacuuming, and when the vacuum reaches 8-10Pa, start to heat up. The plate temperature was raised to -5°C, and th...
Embodiment 2
[0018] Under a 20 lux red light, weigh an appropriate amount of adenosylcobalamin 500mg and mannitol 30000mg, add it to water filled with carbon dioxide gas, stir to dissolve, add 0.1% (g / ml) activated carbon, stir, sample, intermediate Detection, coarse filtration, fine filtration, and packing in brown vials (adenosylcobalamin 5mg / branch). Carbon dioxide gas protection. Arrange them in a freeze-drying box and freeze-dry them. After completion, fill the freeze-drying bottles with carbon dioxide gas and plug them out of the box.
Embodiment 3
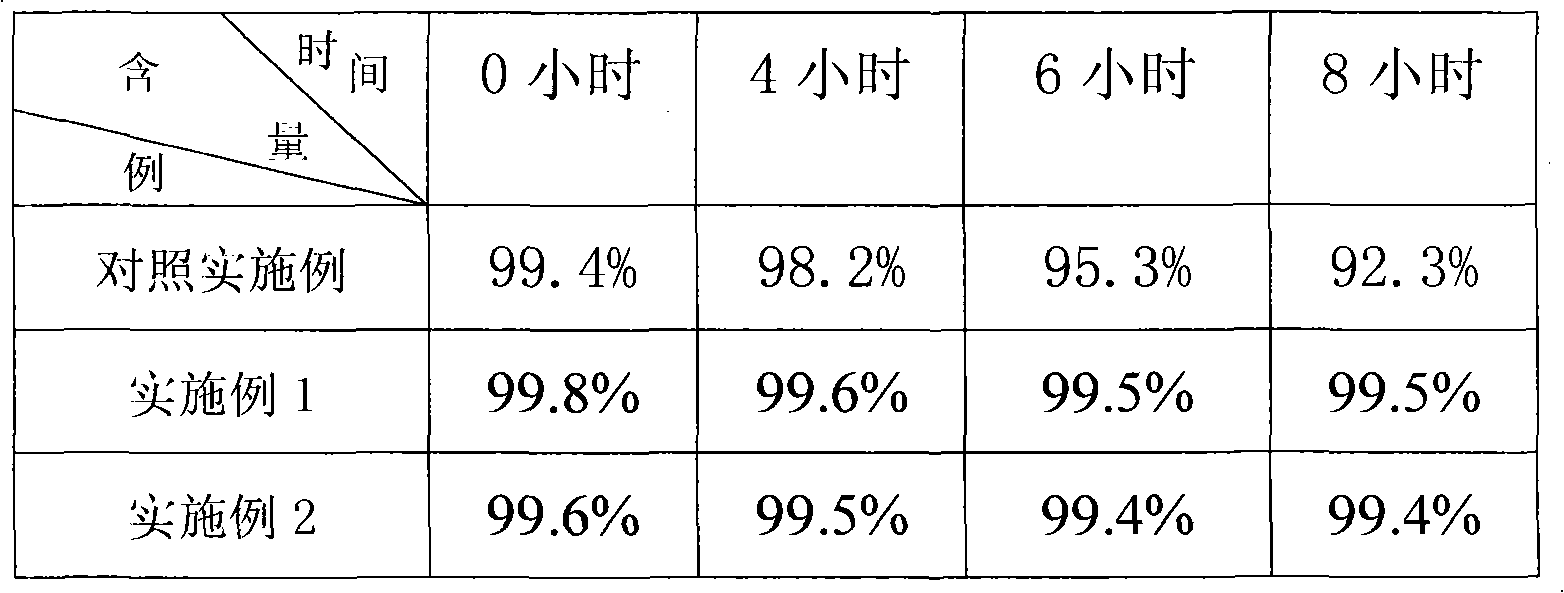
[0022] Example 3 The drug quality stability test prepared by different production methods, the test results are shown in Table 1.
[0023] Table 1
[0024]
[0025] Experimental results show that the quality stability of the product prepared by the method of the present invention is better.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com