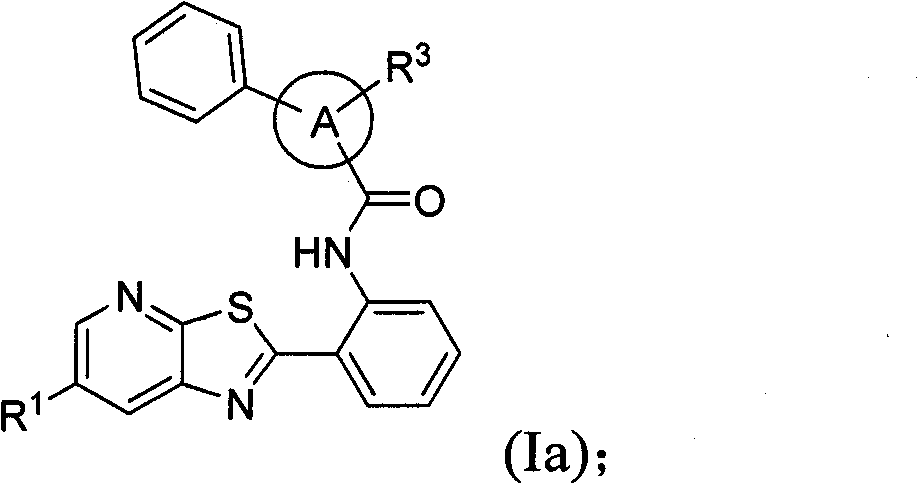
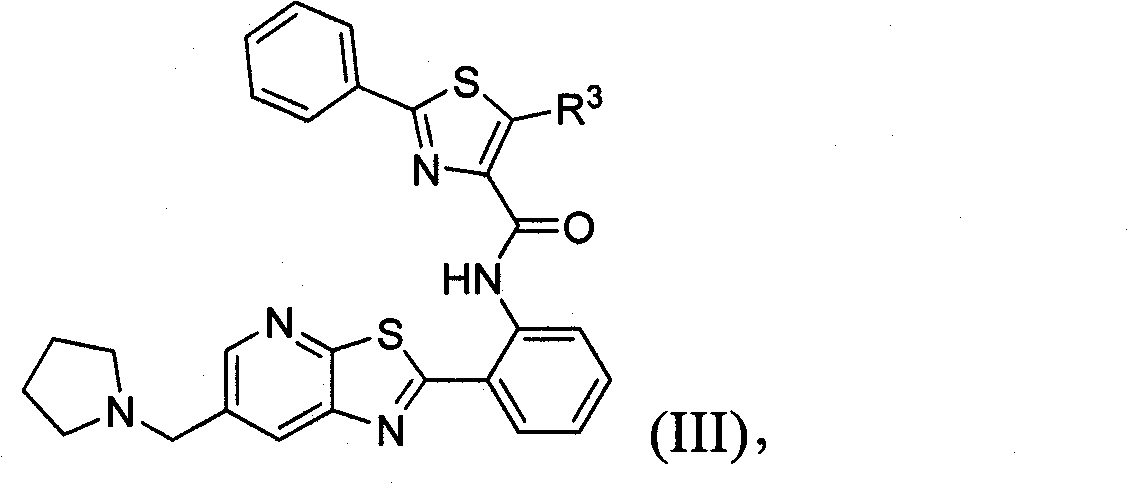
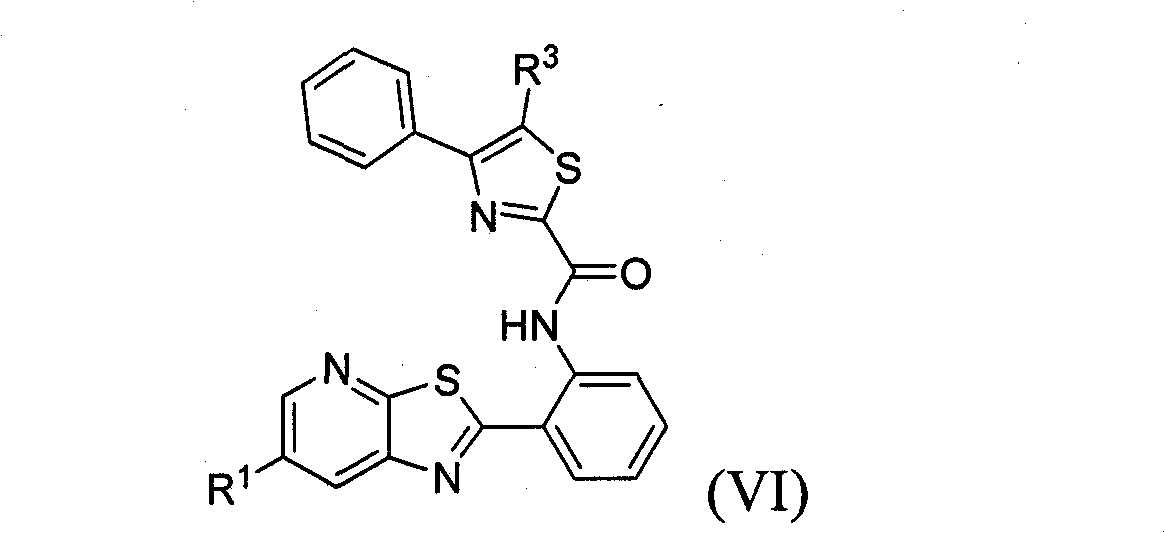
Thiazolopyridine sirtuin modulating compounds
A technology of compounds and compositions, applied in the compound field of thiazolopyridine sirtuin regulators, capable of solving problems such as prolonging the lifespan of wild-type cells and reducing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0286] Example 1. Preparation of sirtuin-modulating compounds and precursors thereof
[0287] Preparation of 2-(thiazolo[5,4-b]pyridin-2-yl)aniline:
[0288]
[0289] A mixture of 3-amino-2-chloropyridine (3.85g, 29.95mmol) and 2-nitrobenzoyl chloride (5.56g, 29.95mmol) in pyridine (50mL) was stirred at 0°C for 1 hour, then at room temperature Stir overnight. Water was added and the formed precipitate was collected by filtration and dried to give N-(2-chloropyridin-3-yl)-2-nitrobenzamide as a white solid (8.52 g, crude yield: >100%) .
[0290] N-(2-chloropyridin-3-yl)-2-nitrobenzamide (12.98g, 46.75mmol), P 2 S 5(31.17 g, 140.24 mmol) and pyridine (80 mL) in p-xylene (310 mL) was heated at 120° C. for 18 hours. Stirring was stopped for 30 min and the mixture was cooled to 100 °C. The upper clear solution was transferred and concentrated in vacuo, followed by the addition of ethanol (50 mL). The suspension was heated at 75°C for 30 minutes to dissolve the product, fi...
Embodiment 2
[0447] Example 2. Biological activity
[0448] Modulators of SIRT1 activity were identified using mass spectrometry-based assays. This mass spectrometry-based assay utilizes the following peptide of 20 amino acid residues: Ac-EE-K(biotin)-GQSTSSHSK(Ac)NleSTEG-K(5TMR)-EE-NH2 (SEQ ID NO: 1), Where K(Ac) is an acetylated lysine residue, and Nle is norleucine. The peptide was C-terminally labeled with the fluorophore 5TMR (excitation 540nm / emission 580nm). The sequence of this peptide substrate is based on p53 with several modifications. In addition, the methionine residue naturally occurring in this sequence was replaced with norleucine, since methionine may be easily oxidized during synthesis and purification.
[0449] Mass spectrometry was performed as follows: 0.5 μM peptide substrate and 120 μM βNAD + With 10nM SIRT1 at 25°C in reaction buffer (50mM Tris-acetate pH 8, 137mM NaCl, 2.7mM KCl, 1mM MgCl 2 , 5mM DTT, 0.05% BSA) for 25 minutes. Test compounds can be added t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com