Symmetrical difunctional coupling agent and coupled molecular imaging agents thereof
A technology of molecules and names, which is applied in the application field of preparing imaging agent drugs, can solve problems such as shortage, achieve the effects of low toxicity and side effects, easy purification, and increase reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
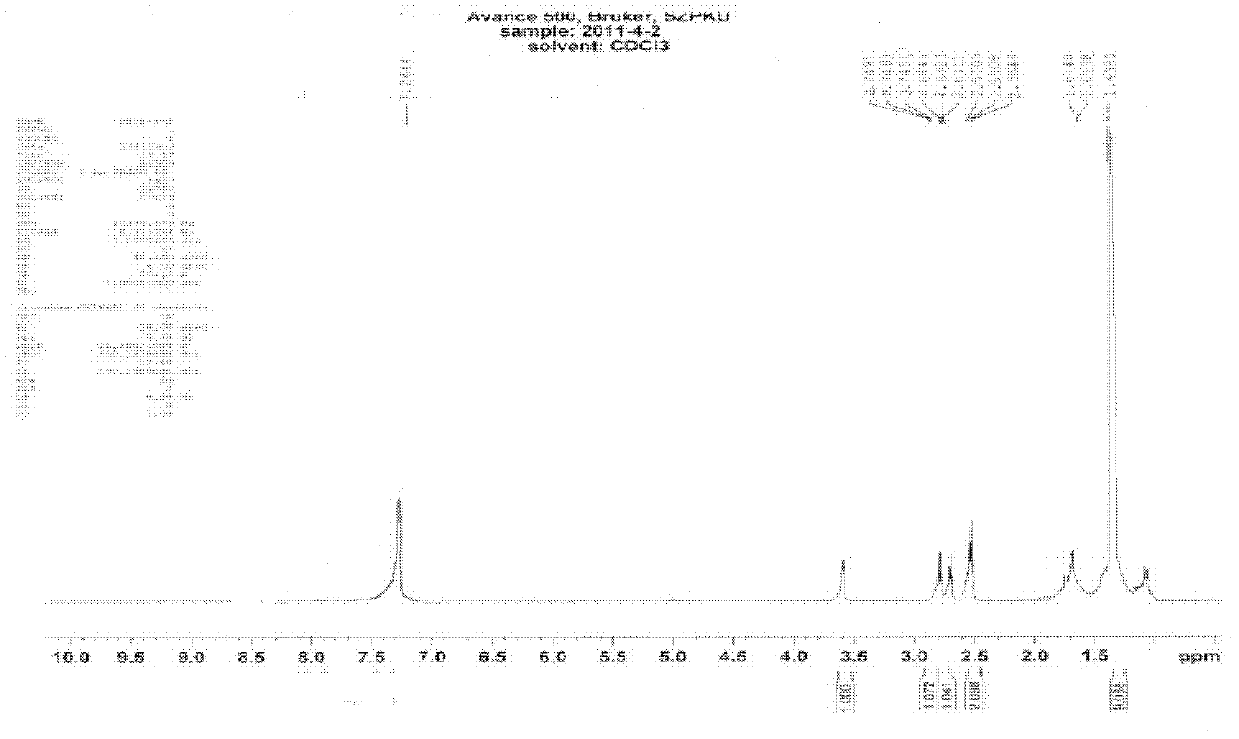
[0106] [Example 1] Symmetrical bifunctional coupling agent N-fluorenylmethoxycarbonyl-L-beta-glutamic acid-two-N-succinimide (Fmoc-beta-Glu(OSu)-OSu, I) Synthesis
[0107] Synthetic Route 1 Symmetrical bifunctional coupling agent N-(9-fluorenylmethoxycarbonyl)-L-beta-glutamic acid-di-N-succinimide (Fmoc-beta-Glu(OSu)-OSu, I) Synthesis.
[0108] ① The compound Fmoc-beta-Glu(OtBu)-OH is used as the raw material, and the compound Fmoc-beta-Glu(OH)-OH is obtained after the tert-butyl group (tBu) is removed by TFA (trifluoroacetic acid) hydrolysis.
[0109]
[0110] ②Fmoc-beta-Glu(OH)-OH reacts with HOSu (N-hydroxysuccinimide) under the action of DCC (dicyclohexylcarbodiimide) to form a symmetrical bifunctional coupling agent Fmoc-beta-Glu( OSu)-OSu(I).
[0111]
[0112] Specific steps are as follows:
[0113]Organic synthesis of coupling group N-(9-fluorenylmethoxycarbonyl)-L-beta-glutamic acid-di-N-succinimide (Fmoc-beta-Glu(OSu)-OSu, I).
[0114] Fmoc-beta-Glu(OSu)-OS...
Embodiment 2
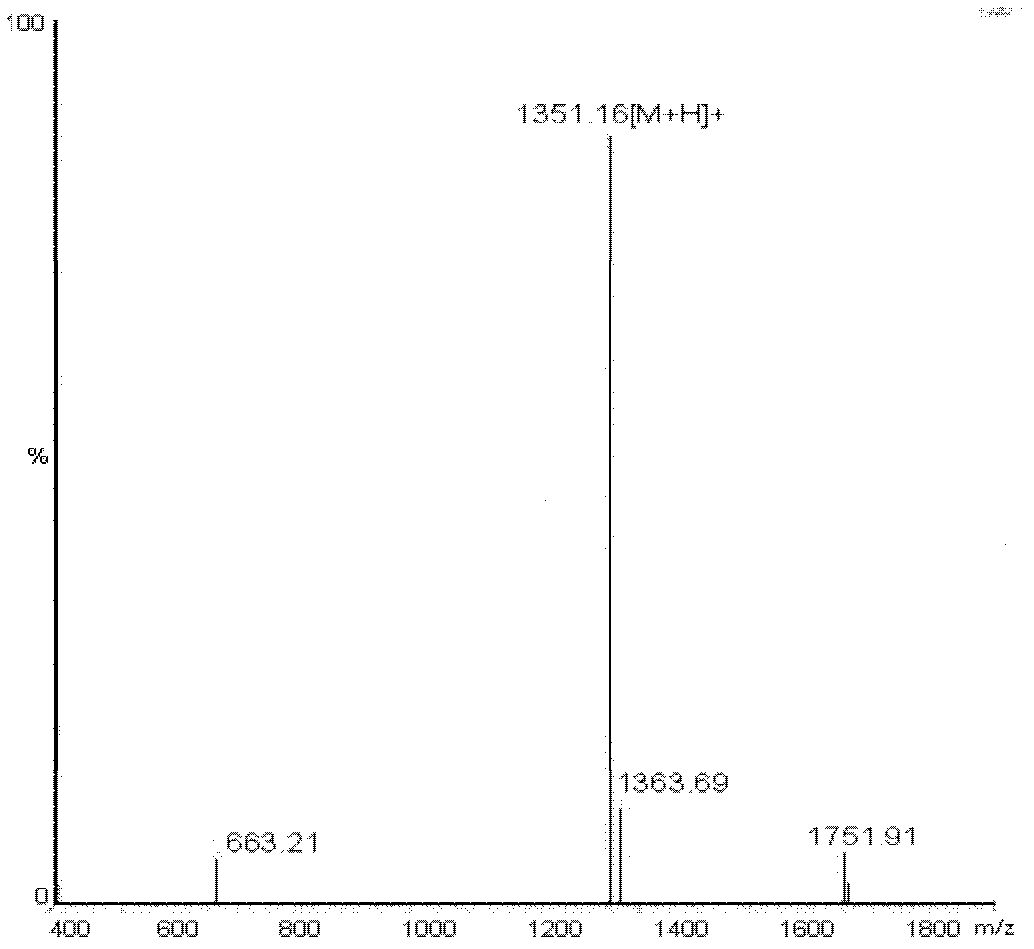
[0115] [Example 2] Synthesis of precursor-coupled bivalent peptide molecule beta-Glu-E(RGDyk)2 and coupled hybrid molecule RGD-beta-Glu-BBN
[0116] Synthetic Route 3 Solid phase synthesis of precursor bivalent peptide molecule beta-Glu-E(RGDyk)2.
[0117] ① Connect 9-fluorenylmethoxycarbonyl-aspartic acid-allyl (Fmoc-Asp-Oall) to wang resin (Wang's resin) to generate resinized product (5).
[0118]
[0119] ② Connect glycine (Gly), 2,2,4,6,7-pentamethylbenzofuran-5-sulfonyl (pbf)-protected arginine (Arg), 1 -(4,4-dimethyl-2,6-dioxocyclohexyl-1-ylidene)ethane (Dde) protected lysine (Lys), and tert-butyl (tBu) protected D- Tyrosine (Tyr), remove allyl group (Oall),
[0120] Cyclization to obtain the resin cyclization product (6). cyclic(RGDyk(Dde)) is a cyclic monomer RGD protected by Dde group.
[0121]
[0122] ③ The resin cyclization product (6) is reduced by hydrazine to remove the Dde group to obtain the cyclization RGD product (7). .
[0123]
[0124] ④The ...
Embodiment 3
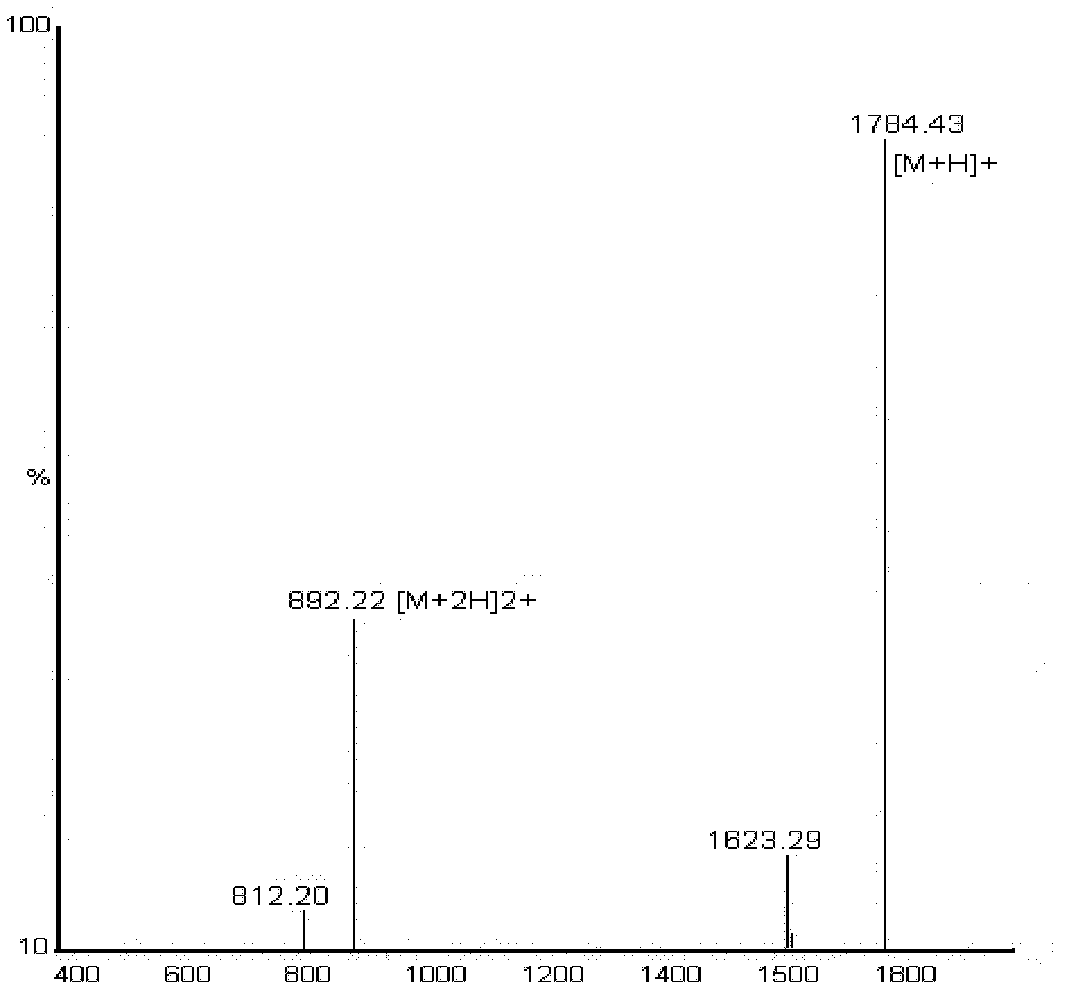
[0142] [Example 3] 18 F labeled prosthetic group N-succinimide-4- 18 F-fluorobenzoate ( 18 F-SFB) radiosynthesis and its use to label beta-Glu conjugated molecules.
[0143] Synthetic Route 5 Reporter Signal Group Nuclide Labeled Prosthetic Group (S): N-Succinimide-4- 18 F-fluorobenzoate ( 18 F-SFB) radiosynthesis.
[0144] ① Using ethyl 4-(trimethylammonium trifluoromethanesulfonate) benzoate (9) as raw material, compound (10) was obtained through fluorination.
[0145]
[0146] ② Compound 10 reacts with tetrapropylammonium hydroxide to generate 4- 18 F-Ammonium benzoate (11).
[0147]
[0148] ③Compound 11 reacts with N,N,N',N'-tetramethyl-0-(N-succinimide) hexafluorophosphate urea salt (HSTU) under the action of tetrapropylammonium hydroxide to generate 18 F-SFB.
[0149]
[0150] Synthetic Route 6 Bivalent Peptide Molecular Imaging Agent 18 Radiosynthesis of F-FB-beta-Glu-E(RGDyk)2. beta-Glu-(RGDyK)2 dissolved in Na 2 HPO 4 or borate buffer, add a smal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com