New medicinal application of andrographolide
A technology of andrographolide and drugs, applied in the field of medicine, can solve the problems of andrographolide in the treatment of malignant glioma and its mechanism of action, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
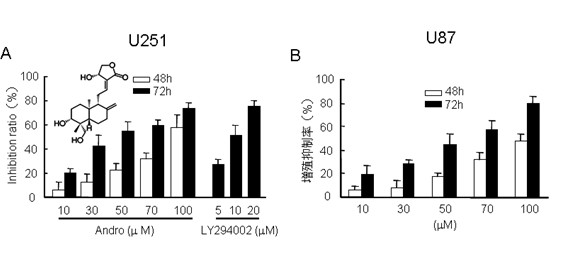
[0016] Example 1. The inhibitory effect of andrographolide on the growth of malignant glioma U251 and U87 cells in vitro
[0017] 1 Experimental method
[0018] U251, U87 cells adherent growth in the medium containing 10% fetal bovine serum, 100U / ml penicillin, 100μg / ml streptomycin, 0.2% NaHCO 3 RPMI-1640 culture medium, placed in 5% CO 2 , saturated humidity, and 37°C incubator, and the culture medium was changed every other day for subculture.
[0019] Take the adherent tumor cells in the logarithmic growth phase and make 6×10 4 cells / mL concentration, 100 μl per well was inoculated in a 96-well plate and cultured overnight. After the cells were fully adhered to the wall, the original culture medium was removed, and different concentrations of andrographolide (provided by the Natural Medicine Research Laboratory of Shenyang Pharmaceutical University), PI3K / Akt signal-specific inhibitor LY294002 (5 μM, 10 μM, 20 μM, positive control) or DMSO (final concentratio...
Embodiment 2
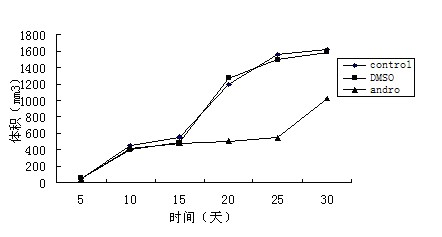
[0023] Example 2. Inhibitory effect of andrographolide on tumor growth in animal model bearing glioma in vivo
[0024] 1 Experimental method
[0025] Fifteen six-week-old female nude mice were purchased from the Chinese Academy of Military Medical Sciences and raised in a sterile system with constant temperature and humidity. Divided into three groups, blank control group, DMSO group and andrographolide (Andro) administration group, 8 rats in each group. Take U251 cells and adjust to a cell concentration of 3×10 7 / ml, the cell viability was detected by trypan blue, and the cell survival rate was >90%. The above suspension was drawn up with a syringe, and 100 μl of the single cell suspension was subcutaneously injected on the lateral side of the right hind limb. Five days after inoculation, the nude mice in the three groups were measured with a vernier caliper to measure the long and short diameters of the tumors, and then the corresponding doses of drugs were given ...
Embodiment 3
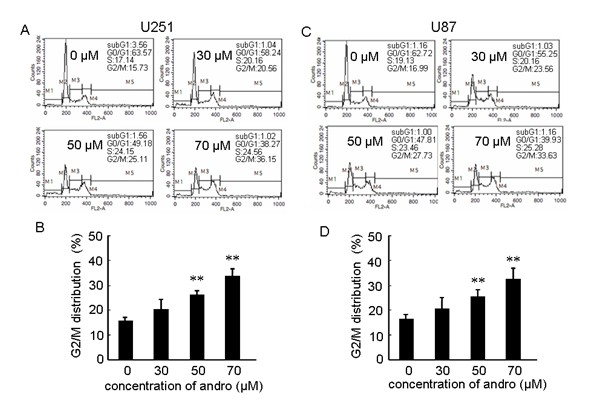
[0028] Example 3, andrographolide can induce malignant glioma U251, U87 cell cycle arrest in G 2 / M phase to inhibit cell growth;
[0029] 1 Experimental method
[0030] In order to confirm the mechanism of andrographolide inhibiting the proliferation of human malignant glioma U251 and U87 cells, the changes of cell cycle distribution after different concentrations of andrographolide were investigated by flow cytometry. U251, U87 cells at 1×10 6 Cells / ml were planted in a culture flask, and the cells were collected 72 hours after adding different concentrations of andrographolide, centrifuged at 1000 rpm / centrifuge for 5 minutes, carefully discarded the supernatant, added 500 μl PBS to suspend the cells, and added 10 ml of 70% ice-cold ethanol and fixed overnight at -20°C. Centrifuge the sample before detection at 1000 rpm / centrifuge for 5 minutes, carefully discard the supernatant, add 10 μl of RNAase (200 μg / ml) to each sample, mix well, and act for 30 minutes at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com