Method for preparing lithium carbonate superfine powder through solvating-out and reaction crystallization
A technology of reaction crystallization and ultra-fine powder, which is applied in the direction of lithium carbonate; High recovery rate and excellent product effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
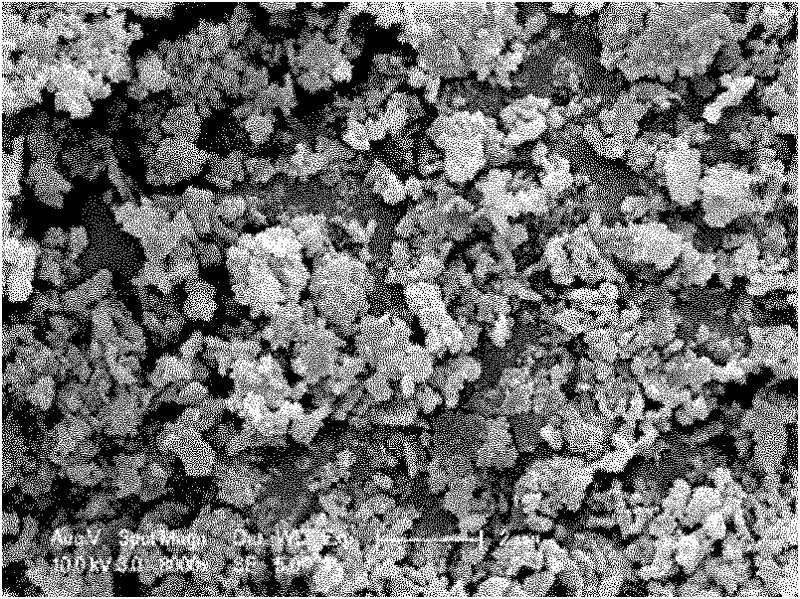
[0026] This embodiment is used to illustrate the method for preparing lithium carbonate ultrafine powder by the dissolution-reaction crystallization method provided by the present invention.
[0027] First prepare LiCl(1~16mol / L)-ethanol-water ternary system solution 50ml and Na 2 CO 3 Solution (1 ~ 4mol / L) 50ml. Then, the two reaction raw materials are quickly mixed and reacted in a 150ml reaction crystallizer under the action of ultrasound and the condition of 5-90°C. Due to the anti-solvent effect of ethanol, lithium carbonate can produce a very high degree of supersaturation and produce a large number of fine crystals. Moreover, ethanol itself is also a dispersant, and there is no need to introduce more other dispersants. The feed solution was filtered and dried to obtain a crystalline product. After the filtrate is rectified, ethanol can be separated for recycling. Experimental result shows, this method can reduce the secondary particle diameter of lithium carbonate ...
Embodiment 2
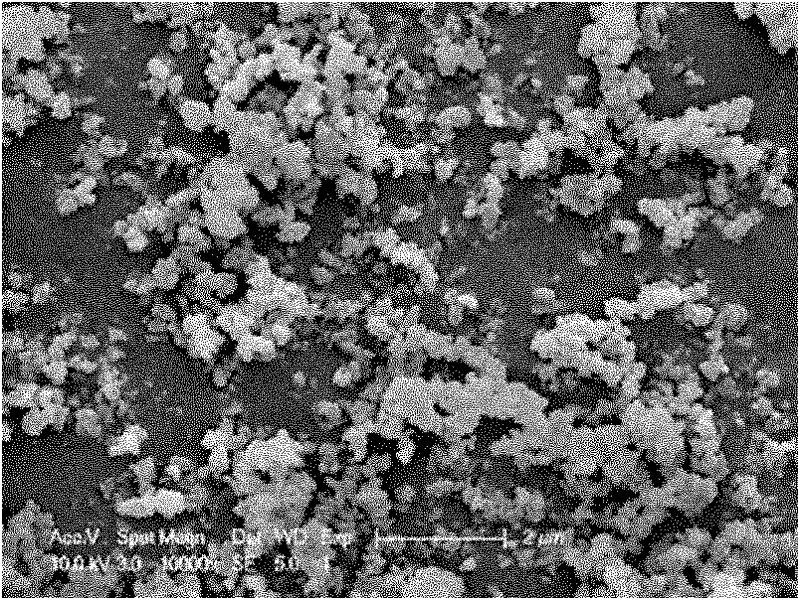
[0029] This embodiment is used to illustrate the method for preparing lithium carbonate ultrafine powder by the dissolution-reaction crystallization method provided by the present invention.
[0030] In the 150ml reaction crystallizer, add LiHCO 3 Solution (0.5~1.5mol / L) 50ml, control LiHCO 3 Under the condition that the temperature of the solution is 5-60° C., an ultrasonic device is introduced and turned on. Into the reaction crystallizer LiHCO 3 Ethanol is added into the solution, and the amount of ethanol added is 5-85%. After adding the dissolving agent, crystallization and precipitation appeared rapidly, and a large number of bubbles were generated in the solution, indicating that the addition of ethanol promoted the crystallization of lithium carbonate, and the crystallization process of lithium carbonate also promoted the formation of LiHCO. 3 Rapid decomposition, this process can be called dissolution-decomposition-reaction crystallization process.
[0031] The fe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com