One-step safety production method of guanidine nitrate
A technology for safe production and guanidine nitrate, which is applied in the chemical industry and can solve problems such as no guidance plan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
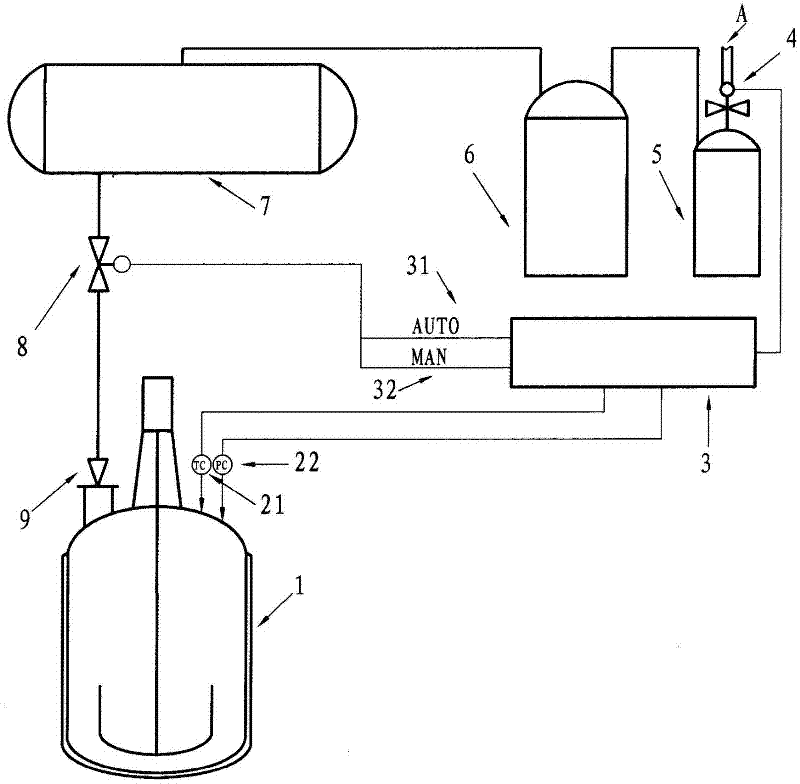
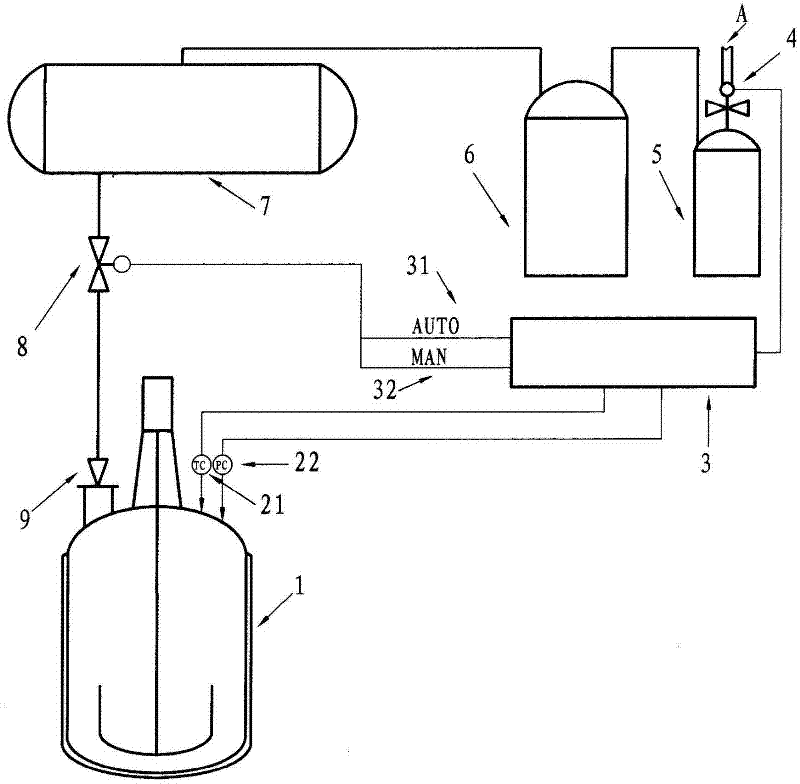
Image
Examples
Embodiment 1
[0011] Example 1: A safe production method for one-step synthesis of guanidine nitrate, which is made into guanidine nitrate products through the following process steps (the main reaction formula is shown below):
[0012]
[0013] ① Add 1500Kg of raw materials ammonium nitrate and 1000Kg of urea and 150Kg of catalyst silica or inorganic silica gel or alumina (aluminum oxide) into a 5000L reactor. The catalyst selected in this embodiment is silica (or alumina). The weight ratio of urea, ammonium nitrate and catalyst used is 1:1.5:0.15. Heating makes the raw material urea and ammonium nitrate become molten liquid (the liquid temperature is between 170~175℃). ② (Through the heat transfer medium) control the temperature of the reaction liquid in the reactor to 200°C (200±5°C can be selected), and keep this temperature for 3 hours. Set the over-temperature alarm value to 206℃ and the pressure alarm value to 0.1MPa gauge pressure. ③After the reaction, start to cool down and cool to 1...
Embodiment 2
[0015] Example 2: A safe production method for the one-step synthesis of guanidine nitrate, which is made into guanidine nitrate products through the following process steps (the main reaction formula is shown in Example 1): ① Add the raw material ammonium nitrate 1000Kg and Urea 1000Kg and catalyst silica or inorganic silica gel or alumina (aluminum oxide) 150Kg, the catalyst selected in this embodiment is alumina (or silica). The weight ratio of urea, ammonium nitrate and catalyst used is 1:1:0.15. Heating makes the raw material urea and ammonium nitrate become molten liquid (the liquid temperature is between 170~175℃). ② (Through the heat transfer medium) control the temperature of the reaction liquid in the reactor to 190℃ (190±5℃ can be selected), and keep this temperature for 5 hours. Set the over-temperature alarm value to 206℃ and the pressure alarm value to 0.1MPa gauge pressure. ③After the reaction, start to cool down and cool to 160°C. (One-time) add 3000L of distil...
Embodiment 3
[0017] Example 3: A safe production method for the one-step synthesis of guanidine nitrate, which is made into guanidine nitrate products through the following process steps (the main reaction formula is shown in Example 1): ① Add the raw materials ammonium nitrate and urea and The catalyst is silica or inorganic silica gel or alumina, and the catalyst selected in this embodiment is inorganic silica gel (or silica). The weight ratio of urea, ammonium nitrate and catalyst used is 1:2.0: 0.2 (or 1:1.2: 0.1), and the raw materials urea and ammonium nitrate become molten liquid by heating. ② Control the temperature of the reaction liquid in the reactor at 180°C (or 220°C), and keep this temperature for 1 hour (or 6 hours). Set the over-temperature alarm value to 206℃ and the pressure alarm value to 0.1MPa gauge pressure. ③After the reaction, the temperature is reduced to 170°C (or 100°C), and process water is added. The ratio of the weight of urea plus ammonium nitrate to the weig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com