Recombined membrane-penetrating peptide with WNK (with no lysine (K)) kinase inhibiting effect
A kinase inhibition, membrane-penetrating peptide technology, applied in recombinant DNA technology, medical preparations containing active ingredients, peptides, etc., can solve problems such as poisoning and death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Preparation of TAT-LIC peptide
[0017] The LIC peptide (LINGO-1, intracellular domain) corresponds to the amino acid sequence 580-620 of the human LINGO-1 molecule, and is derived from a subclone using the full-length structure pEGFP-N1-hLINGO-1 as a template. The production of the fusion protein TAT-LIC peptide is to correctly insert the smallest translocation domain sequence (MRGSHHHHHHGMARGYGRKKRRQRRRPQ) in the HIV1 Tat protein into the N-terminus of the corresponding LIC cDNA. The fusion protein was expressed by BL21 system in vitro and purified by nickel-affinity chromatography column according to the classical method.
[0018]
Embodiment 2
[0019] Example 2: Culture of dorsal root ganglion neurons in SD rat spinal cord
[0020] Extraction and culture of dorsal root ganglion of the spinal cord Aseptically remove embryos from E16 pregnant mice after ether anesthesia, put them into 4 ℃ PBS solution, expose the spinal canal and intervertebral foramen of SD fetal mice under a dissecting microscope, and use forceps to remove the embryos of the spinal cord Take out the DRGs on both sides and put them into PBS solution, remove the DRGs one by one with a microscope, and peel off the fascia on the surface of the ganglion as much as possible, put it into a 35mm culture dish, cut the ganglion into pieces with ophthalmic scissors, and transfer it into 2mL 0.25% In the centrifuge tube of trypsin digestion solution, digest in a CO2 incubator at 37 ℃ for 20 minutes, suck off the digestion solution, directly add DMEM medium containing 10% FBS to terminate the digestion reaction, discard the supernatant after centrifugation, and re...
Embodiment 3
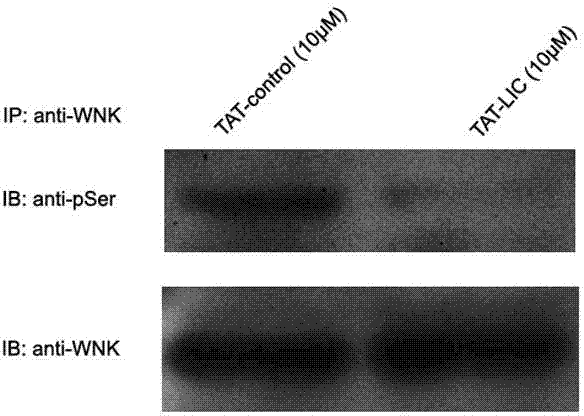
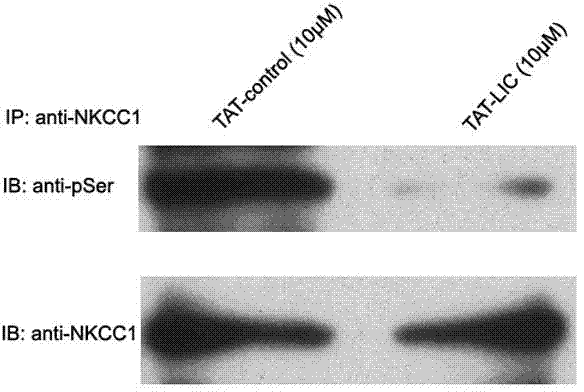
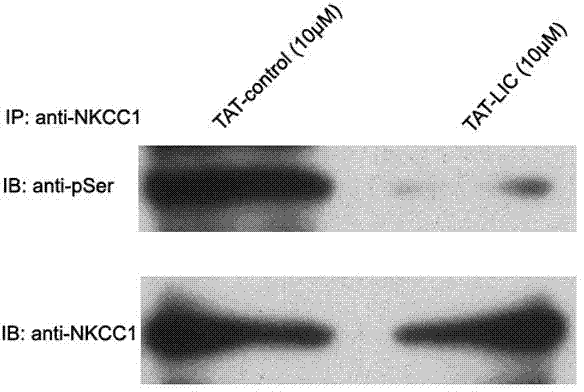
[0021] Example 3: Treating primary cultured spinal cord dorsal root ganglion neurons of SD rats with TAT-LIC peptide can significantly inhibit the phosphorylation of WNK kinase, that is, inhibit the activity of WNK kinase. (See figure 1 )
[0022] The specific experimental process is as follows: the primary cultured spinal cord dorsal root ganglion neurons of SD rats obtained in Example 2 were treated with TAT-LIC peptide (final concentration: 100nM) or control peptide (final concentration: 100nM) for 3 hours . Wash twice with PBS and add RIPAI tissue lysate (20mM Tris-HCl, pH 7.5, 137mM NaCI, 50mM NaF, 0.02%NaN3, 1%NP-40, 2mM EDTA, 2mM benzamidine, 1mM PMSF, 2pg / ml pepstatin A, 2pg / ml leupeptin, 4 pg / ml antipain, 2pg / ml aprotinin, 2mM DTT) and phosphatase inhibitors. The cells were manually homogenized on ice, and the homogenate was centrifuged at 14,000 rpm for 10 min at 4°C, and the supernatant was collected. Co-immunoprecipitation and Western blot detection: Take 500 μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com