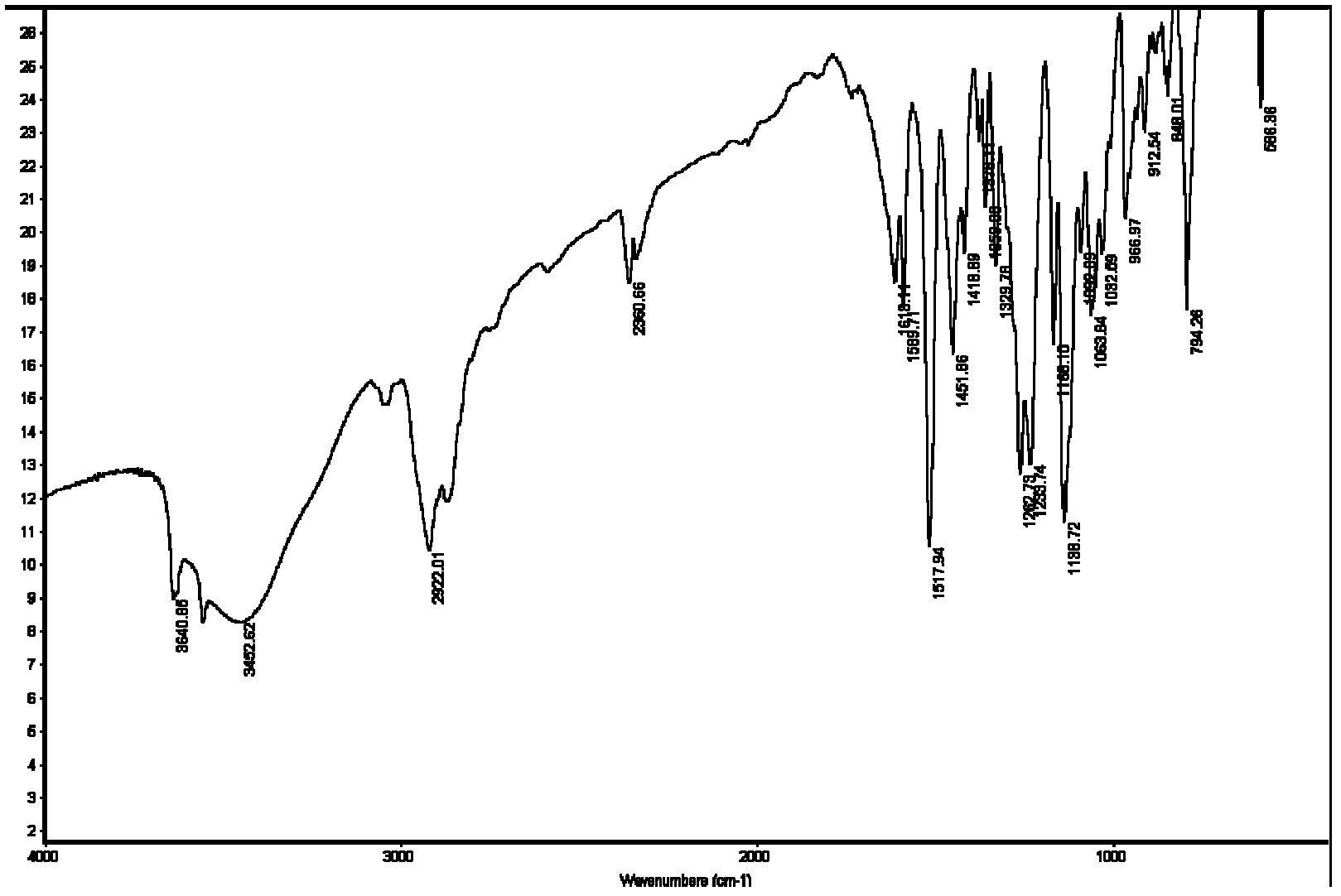
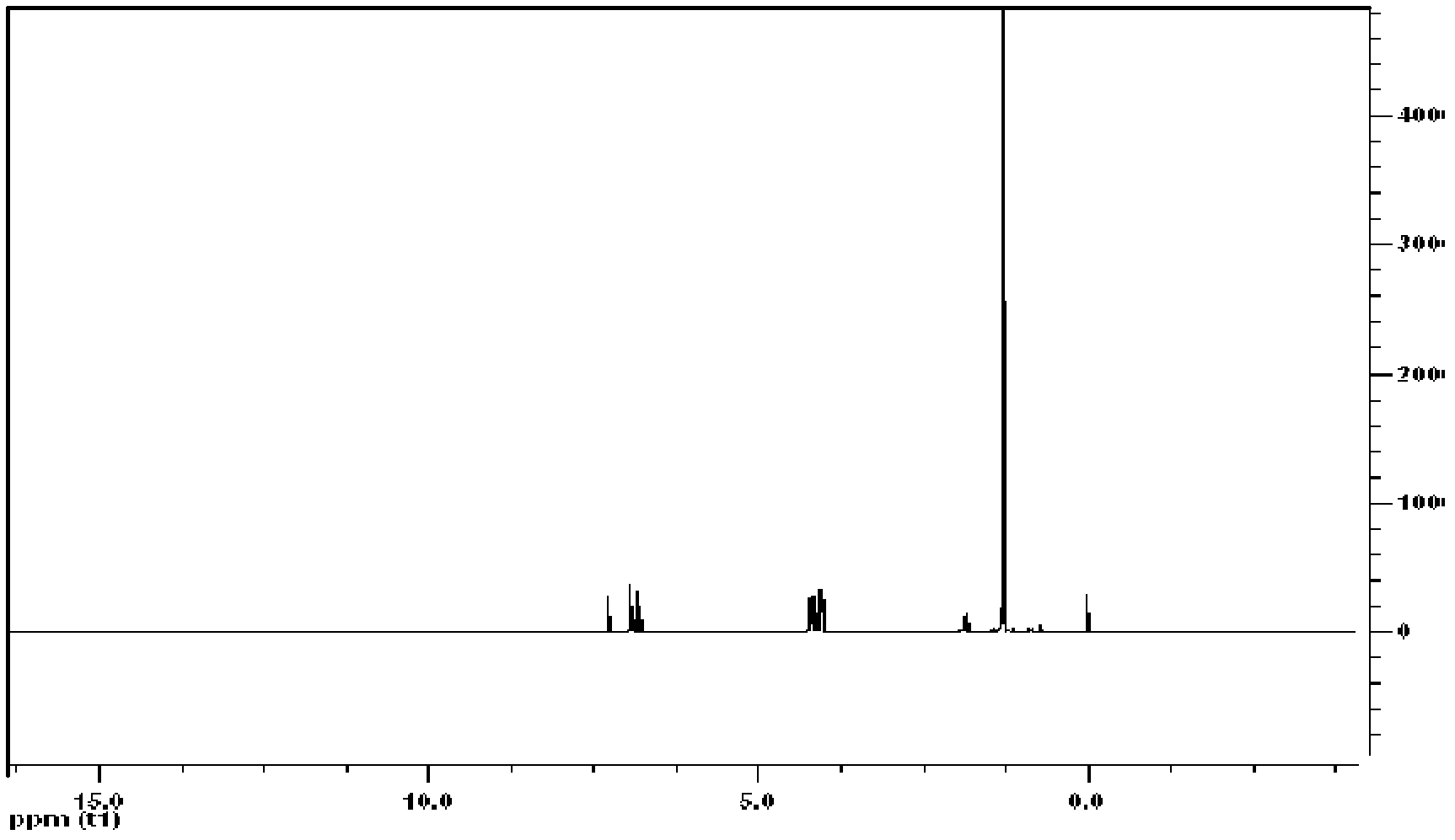
Extracting agent for nuclear waste liquid and preparation method for extracting agent
A technology of extractant and nuclear waste liquid, which is applied in the field of nuclear waste liquid extractant and its preparation, can solve the problems of low yield, difficulty in separation and purification, and limitation of large-scale application, and achieve the goal of increasing conversion rate and simplifying the separation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] a. Weigh 6.2 g (0.05 mol) of p-methylcatechol and 32.5 g (0.1 mol) of cesium carbonate and dissolve them in 200 ml of tetrahydrofuran, wherein the cesium carbonate is added in two equal amounts. Weigh 0.05 mol of diethylene glycol xylene sulfonate, dissolve in 100 ml of tetrahydrofuran twice, and add dropwise in a constant pressure dropping funnel. The reaction was carried out at 40°C for 24 hours, and the reaction conversion rate was 26.3%.
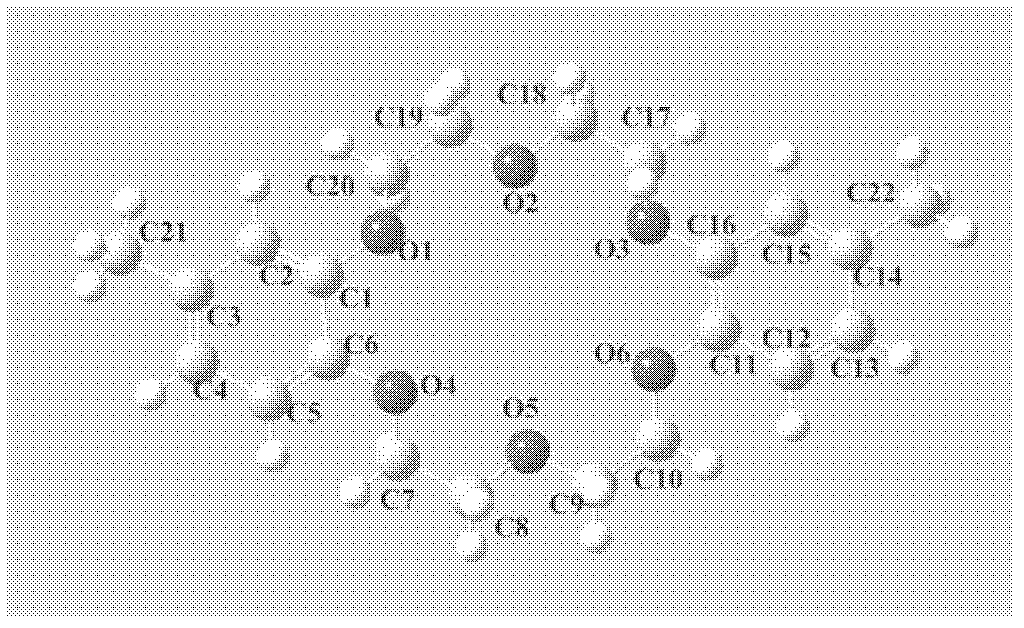
[0023] b. Filtrate the mixture obtained by reacting according to the above step a, and distill under reduced pressure. Take 5g of the mixture, add 500g of γ-alumina and 500ml of n-heptane, reflux and co-heat the reaction at 60°C for 6h, filter the filtrate, and vacuum distill the filtrate. Recrystallization gave 4', 4"-dimethyldibenzo-18-crown-6( image 3 ), yield 50.5%, purity 73.6%.
Embodiment 2
[0025] a. Weigh 6.2 g (0.05 mol) of p-methylcatechol and 65 g (0.2 mol) of cesium carbonate and dissolve them in 300 ml of tetrahydrofuran, wherein the cesium carbonate is added in two equal amounts. Weigh 0.075 mol of diethylene glycol xylene sulfonate and dissolve it in 200 ml of tetrahydrofuran in two equal amounts, and add dropwise in a constant pressure dropping funnel. The reaction was carried out at 60°C for 60 hours, and the reaction conversion rate was 57.6%.
[0026] b. Filter the mixture obtained according to the above step a, weigh 5g of the mixture after vacuum distillation, add 300g of gamma alumina and 300ml of n-heptane, reflux at 70°C for 4h, filter and take the filtrate, and vacuum distill the filtrate. Recrystallization gave 4', 4"-dimethyldibenzo-18-crown-6( image 3 ), yield 54.6%, purity 65.2%.
Embodiment 3
[0028] a. Weigh 6.2g (0.05mol) of p-methylcatechol and 71.25g (0.25mol) of cesium carbonate and dissolve them in 400ml of tetrahydrofuran, wherein the cesium carbonate is added in two equal amounts. Weigh 0.085 mol of diethylene glycol xylene sulfonate and dissolve it in 250 ml of tetrahydrofuran in two equal amounts, and add dropwise in a constant pressure dropping funnel. The reaction was carried out at 50°C for 60 hours, and the reaction conversion rate was 49.5%.
[0029] b. filter the mixture obtained according to the above step a, weigh 5g of the mixture after distillation under reduced pressure, add 250g of thin-layer chromatography silica gel and 300ml of n-hexane, reflux and co-heat for 6h at 60°C, filter the filtrate, and vacuum distill the filtrate. Recrystallization gave 4', 5"-dimethyldibenzo-18-crown-6( Figure 4 ), yield 47.1%, purity 70.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com