Preparation method of alkylphenol medicine analogue NPA
A technology of nonanoic acid phenol and alkylphenol, which is applied in the preparation of organic compounds, carboxylate salts, chemical instruments and methods, etc., can solve the problems of long detection time, expensive instruments, complicated processing, etc., and meet the requirements of immune research needs, the synthesis steps are simple, and the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
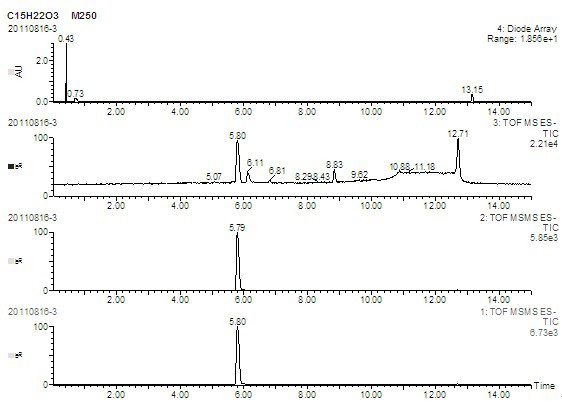
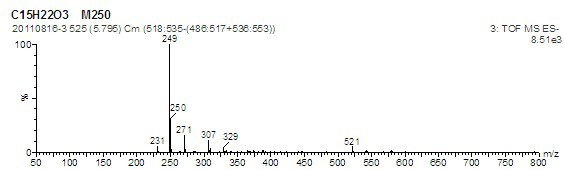
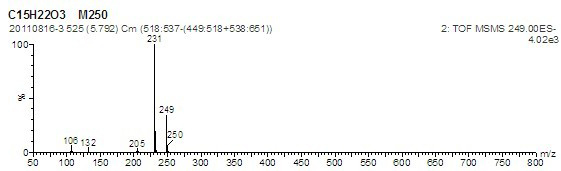
Image
Examples
Embodiment 1
[0029] The process preparation steps of nonanoic acid phenol NPA:
[0030] (1) Synthesis of compound 2:
[0031] Add compound 1 azelaic acid (353 mg, 1.875 mmol) and compound 1′ dimethyl azelate (405 mg, 1.875 mmol) into a 25 mL round bottom flask, then add 5 mL tetrahydrofuran THF, stir overnight at room temperature, after the reaction is complete , the reaction solution was concentrated to obtain the crude product which was directly used in the next reaction (0.75 g, yield: 100%).
[0032] (2) Synthesis of Compound 3:
[0033] Add methyl hydrogen azelate (0.75 g, 3.71 mmol) and thionyl chloride (1.5 mL) into a 25 mL round bottom flask, add two drops of DMF dropwise to the reaction solution, and heat the reaction solution to reflux for two hours. The reaction solution was distilled to remove thionyl chloride, and dichloromethane was used to take out residual thionyl chloride. Compound 3 was obtained (0.82 g, yield: 100%).
[0034] (3) Synthesis of Compound 4:
[0035] Ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com