Method for preparation of antiviral composition and application of intermediate thereof
A technology for intermediates and compounds is applied in the application field of preparing antiviral compositions and intermediates thereof, and can solve the problems of poor selectivity, large reaction volume ratio, and high cost of triphenylphosphine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
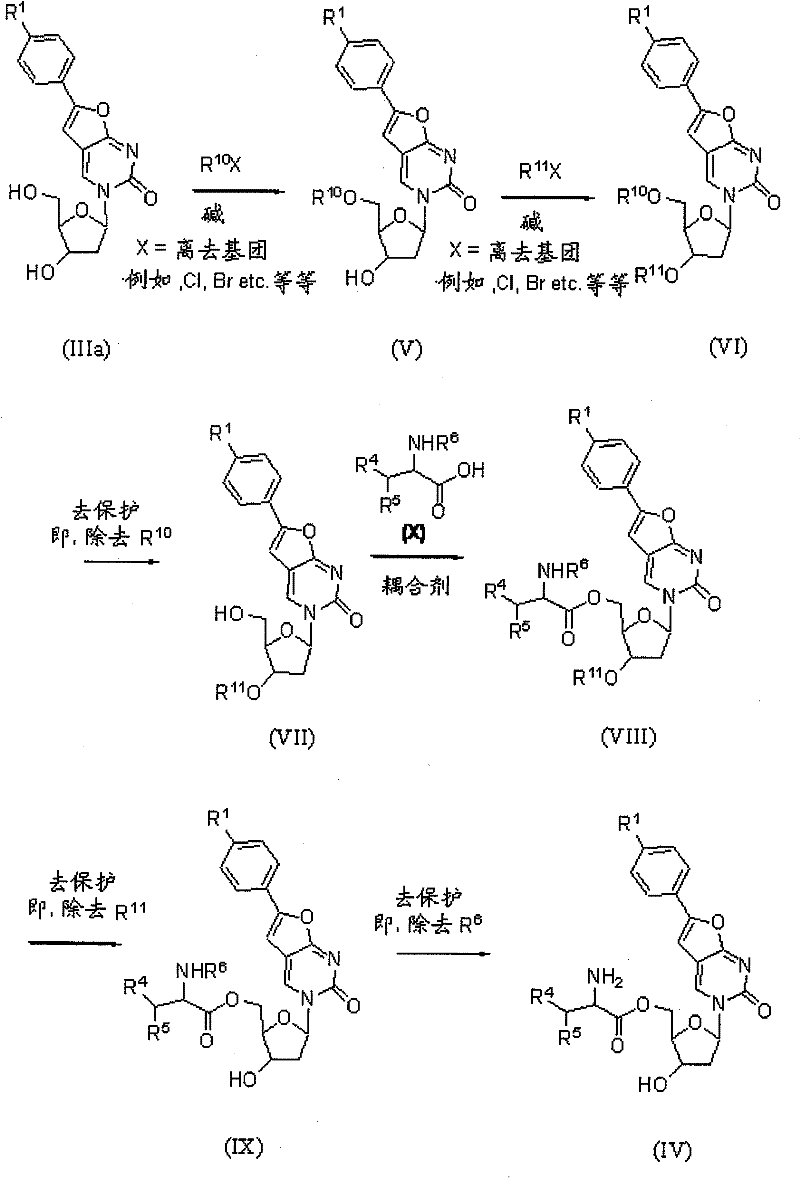
[0256] 3-((2R,4S,5R)-5-(triphenylmethoxy)methyl)-4-hydroxy-tetrahydrofuran-2-yl)-6-(4-pentylphenyl)furan[2 ,3-d]pyrimidin-2(3H)-one
[0257]
[0258] 500 grams (1.25 moles) of 3- ((2R, 4S, 5R) -(4-Hydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-6-(4-pentylphenyl)furan[2,3-d]pyrimidin-2(3H)-one and 2500 Milliliters of pyridine was added to a 5-liter three-necked flask. The mixture was stirred, and 508 g (1.5 mmol) of trityl chloride dissolved in 120 ml of dichloromethane solution was added dropwise thereto at room temperature. After completion of the dropwise addition, the mixture was stirred at room temperature for 3-5 hours. The reaction was then quenched with 50 mL of water. The reaction solution was concentrated to dryness. The residue was dried three times with 500 ml of toluene. The residue was redissolved in 5000 ml of dichloromethane. The organic solution was washed three times with 2500 ml of concentrated brine. The organic layer was dried with 273 g of anhydrous...
Embodiment 2
[0260] 3-((2R,4S,5R)-5-((bis(4-methoxyphenyl)(phenyl)methoxy)methyl)-4-hydroxy-tetrahydrofuran-2-yl)-6- Preparation of (4-pentylphenyl)furan[2,3-d]pyrimidin-2(3H)-one
[0261]
[0262] Add 2.8 kg (7.03 moles) of 3-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-6-(4-pentane) to a 50-liter reactor Phenyl)furan [2,3-d]pyrimidin-2(3H)-one, 2.8 kg (35.4 moles) of pyridine and 22.4 kg of methylene chloride. 2.86 kg (8.44 mol) of 4,4'-dimethoxytrityl chloride (DMT-Cl) was dissolved in 14.9 kg of dichloromethane and dropped into the above mixture while stirring at room temperature. After dripping, the mixture was stirred at room temperature for 0.5 hour. The reaction solution was filtered and the filter cake was washed with 2.5 kg of dichloromethane. The filtrate was washed with 28 kg of 5% sodium bicarbonate aqueous solution. The aqueous layer was extracted with 3.7 kg of dichloromethane. The organic layers were combined and washed with brine. The organic layer was...
Embodiment 3
[0266] 3-((2R,4S,5R)-(5-((tert-butyldimethylsiloxy)methyl)-4-hydroxy-tetrahydrofuran-2-yl)-6-(4-pentylphenyl ) Preparation of furan[2,3-d]pyrimidin-2(3H)-one
[0267]
[0268] Add 398 mg (1.0 mmol) of 3- ((2R, 4S, 5R) (4-Hydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-6-(4-pentylphenyl)furan[2,3-d]pyrimidin-2(3H)-one, 450 mg (3.0 mmol) tert-butyldimethylchlorosilane, 204 mg (3.0 mmol) imidazole, and 5 mL dimethylformamide (DMF). The reaction mixture was stirred at room temperature for 2 hours and monitored by thin layer chromatography (TLC).
[0269] TLC: eluent: petroleum ether / ethyl acetate=1:1;
[0270] 3-((2R,4S,5R)-(4-hydroxy-5-(hydroxymethyl)-tetrahydrofuran-2-yl)-6-(4-pentylphenyl)furan[2,3-d]pyrimidine -2(3H)-ketone:
[0271] R f =0;
[0272] 3-((2R,4S,5R)-(5-((tert-butyldimethylsiloxy)methyl)-4-hydroxy-tetrahydrofuran-2-yl)-6-(4-pentylphenyl )Furan[2,3-d]pyrimidin-2(3H)-one:
[0273] R f =0.25
[0274] Pour the mixture into water. Extract with ethyl acetate. The organic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com