Medicinal agent
A reagent, sodium chloride technology, applied in the field of pharmaceutical reagents, can solve the problem of not being able to stimulate cartilage metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
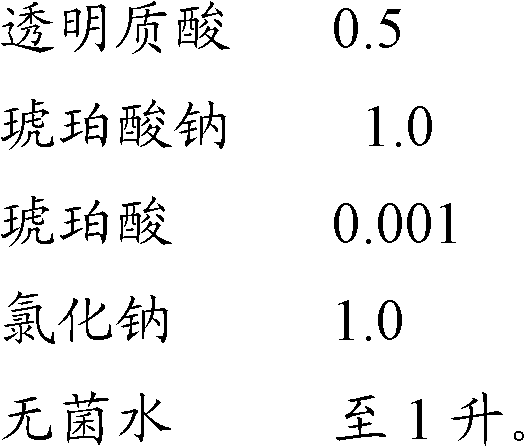
[0031] drug composition
[0032]
Embodiment 3
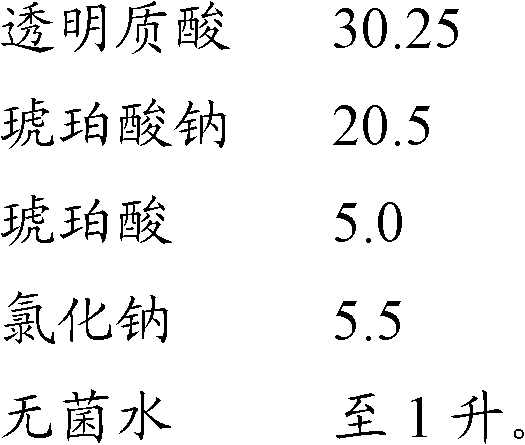
[0034] drug composition
[0035]
[0036]
[0037] The drug was prepared by the following method.
[0038] The following components were used as raw materials:
[0039] - hyaluronic acid (sodium hyaluronate) (Pharm. Eur. 4, 01 / 2002: 1472, P. 1915);
[0040] - succinic acid (P.1228 USP 28);
[0041] - sodium chloride (State Pharmacopeia of Ukraine: 2004, Appendix 1, P.422);
[0042] - Sterile water (State Pharmacopeia of Ukraine: 2004, Appendix 1, P.307).
[0043] Fill the reservoir with the appropriate amount of sodium chloride, sodium succinate, succinic acid, dilute to the required amount with sterile water, and mix well. The calculated amount of hyaluronic acid was added to the resulting solution; it was stirred for 2 hours until a homogeneous mass was obtained.
[0044] Perform sample splitting and analysis; the pH of the solution should be between 5.0 and 8.5, and in 1 ml of the prepared solution, the quantitative content of the formulation components should co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com