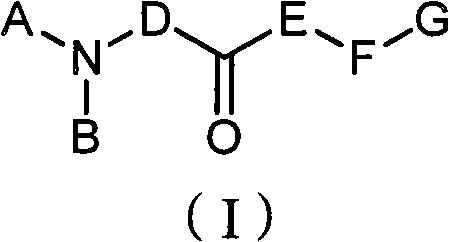
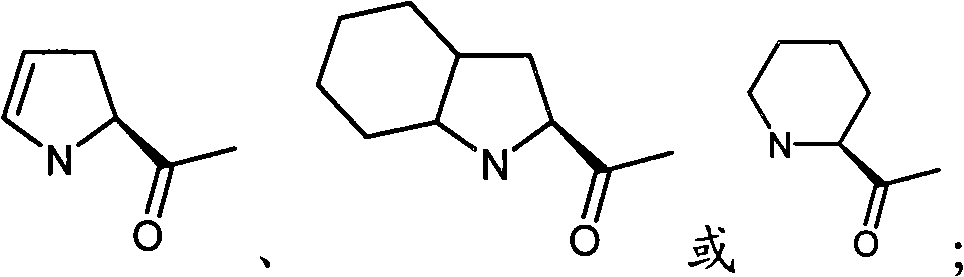
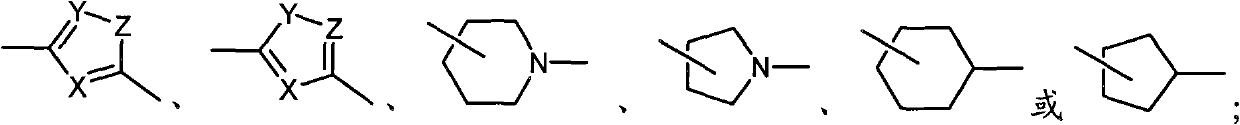New type compound, its preparation method and application
A compound and prodrug technology, applied in the field of new pharmaceutical compounds, can solve the problems of poor oral bioavailability, short half-life, high inhibitory thrombin activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] Embodiment 1: Synthesis of N-benzenesulfonyl-D, L-leucyl-L-prolyl-{[4-(N'-hydroxyl) formamidinophenyl]methyl}amide
[0203] a) N-benzenesulfonyl-D, the preparation of L-leucine
[0204] Dissolve D, L-leucine (2g) in 1.5N sodium hydroxide solution (10ml), add dioxane (15ml), cool and control the temperature at 0-5°C; slowly add benzenesulfonyl chloride (3g) dropwise and 1.5N sodium hydroxide solution to maintain a pH value of 9 to 10; react at 0 degrees for 2 hours, and naturally rise to room temperature for 2 hours. Under cooling, dilute hydrochloric acid was added dropwise to adjust the pH to 3, concentrated under reduced pressure to remove dioxane, and a large amount of solid precipitated; filtered, and the obtained solid was recrystallized with ethyl acetate / petroleum ether to obtain 3.7 g of white solid. Content 99% (HPLC, mobile phase 1, method 2).
[0205] Rf=0.8 developing agent n-butanol: water: acetic acid: ethyl acetate = 1:1:1:1 color development: ultraviol...
Embodiment 2
[0221] Embodiment 2: N-benzenesulfonyl-D, the preparation of L-leucyl-L-prolyl-[(4-formamidinophenyl) methyl] amide acetate
[0222] The compound obtained in Example 1 was dissolved in acetic acid (7ml), acetic anhydride (277mg) and 10% palladium carbon (150mg) were added, hydrogen was passed for 24 hours, filtered, concentrated under reduced pressure to remove the solvent, ether (20ml) was added, Place in the refrigerator overnight, the solid precipitated, filtered and dried (250mg, 66%)
[0223] MS: 560(M+H)
Embodiment 3
[0224] Example 3: N-benzylsulfonyl-D, L-leucyl-L-prolyl-{[4-(N'-hydroxyl) formamidinophenyl]methyl}amide
[0225] a) N-benzylsulfonyl-D, the preparation of L-leucine
[0226] Dissolve D,L-leucine (2g) in 1.5N sodium hydroxide solution (10ml), add dioxane (15ml), cool and control the temperature at 0-5°C; slowly add benzylsulfonyl chloride (3.2g ) and 1.5N sodium hydroxide solution to maintain a pH value of 9 to 10; react at 0 degrees for 2 hours, and naturally rise to room temperature for 2 hours. Under cooling, dilute hydrochloric acid was added dropwise to adjust the pH to 3, concentrated under reduced pressure to remove dioxane, and a large amount of solid precipitated; filtered, and the obtained solid was recrystallized with ethyl acetate / petroleum ether to obtain 3.5 g of white solid. Content 99% (HPLC, mobile phase 1, method 2).
[0227] Rf=0.8 developing agent n-butanol: water: acetic acid: ethyl acetate = 1:1:1:1 color development: ultraviolet, iodine and 1% ninhydri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com