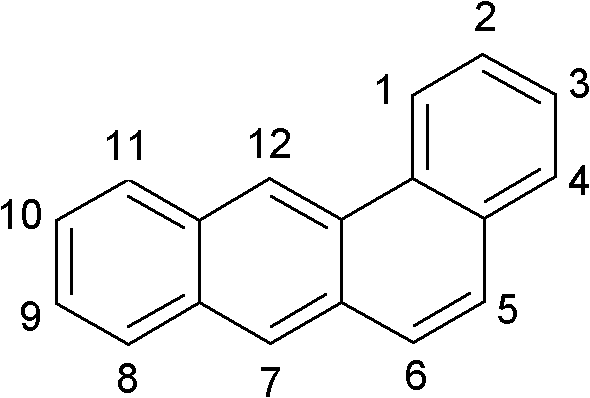
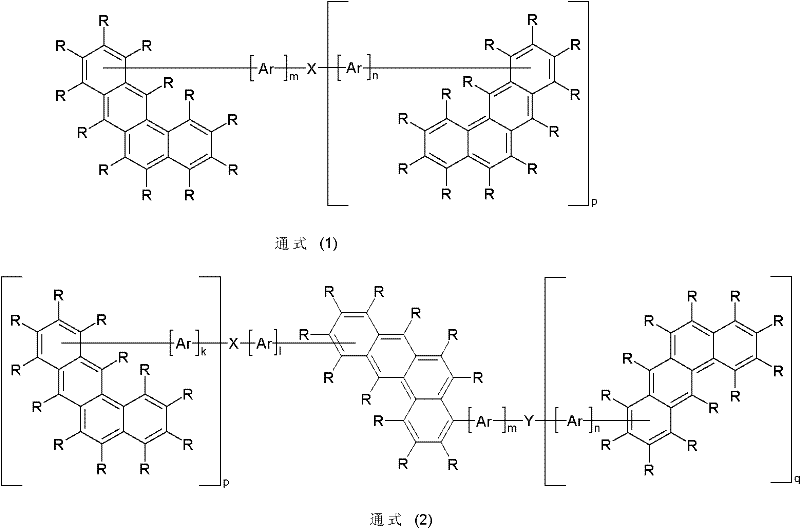
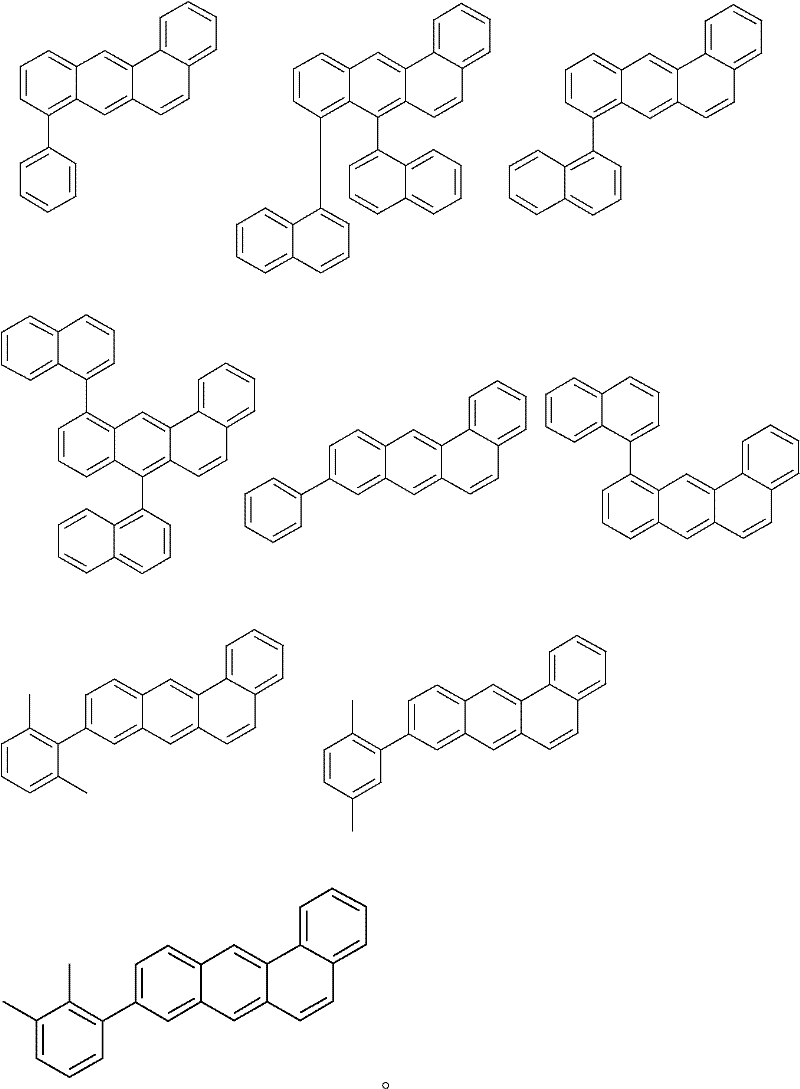
Novel materials for organic electroluminescent devices
A group and general formula technology, applied in the field of organic electroluminescent devices, can solve problems that have not been raised
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Embodiment 1: the synthesis of 8-bromobenzo [a] anthracene
[0180]
[0181] 200ml of hypophosphorous acid (50%) was added to a suspension of 75g (200.2mmol) 8-bromobenzo[a]anthracene-7,12-dione in 900ml of glacial acetic acid, and 400ml of hydroiodic acid (57%) was added dropwise . The reaction mixture was heated to reflux for 16 hours. After cooling, the precipitated solid was filtered off with suction and washed with 500 ml each of water, ethanol / water (1:1, v / v) and ethanol. The crude product was washed by boiling in ethanol and then recrystallized from toluene / glacial acetic acid (1:1, v / v, about 15 ml / g). Drying yielded 35.8 g (115.4 mmol) of 57.6% product with a purity of 99%.
Embodiment 2
[0182] Embodiment 2: Synthesis of benzo [a] anthracene-8-boronic acid
[0183]
[0184] 52ml (130mmol) of n-butyllithium (2.5M in n-hexane) was added dropwise under vigorous stirring at -78°C to a suspension of 30.7g (100mmol) of 8-bromobenzanthracene in 1000ml of tetrahydrofuran. The mixture was stirred for another 2h. 16.7 ml (150 mmol) trimethyl borate was added in one portion to the red solution under vigorous stirring, the mixture was stirred at -78 °C for an additional 30 minutes, then allowed to warm to room temperature over 3 h, and added 300ml of water, and the mixture was stirred for 30 minutes. The organic phase was separated off and evaporated to dryness under vacuum. The solid was dissolved in 100 ml of n-hexane, filtered with suction, washed once with 100 ml of hexane and dried under vacuum. Boronic acid yield: 23.7 g (87 mmol), 87%, about 90% pure (NMR), with varying amounts of boric anhydride and boric acid. Boronic acid can be used in this form without ...
Embodiment 5
[0187] Example 5: Synthesis of 9-(naphthalene-2-yl)-10-(benzo[a]anthracene-8-yl)anthracene
[0188]
[0189] Add 913mg (3mmol) of tri-o-tolylphosphine, then 112mg (0.5mmol) of palladium(II) acetate into well-stirred 19.2g (50mmol) of 9-bromo-10-(2-naphthyl)anthracene, 15.0g (55mmol ) benzo[a]anthracene-8-boronic acid, 25.5g (120mmol) tripotassium phosphate in 300ml toluene, 100ml di a suspension in a mixture of alkanes and 400 ml of water, and the mixture was heated to reflux for 16 hours. After cooling, the precipitated solid was filtered off with suction, washed three times with 50 ml of toluene, three times with 50 ml of ethanol:water (1:1, V:V), and three times with 100 ml of ethanol, which was removed from chlorobenzene ( about 10ml / g), recrystallized three times, then sublimed twice (p=5×10 -5 mbar, T=340°C). Yield: 14.8 g (27.5 mmol), 55.0%, purity 99.9% (HPLC).
[0190] The following compounds according to the invention (Examples 6 to 11) were obtained analogou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com