Method for preparing biaryl compound in pure water
A technology of compound and pure aqueous solution, which is applied in the field of preparing biaryl compounds, to achieve the effects of solving reaction difficulties, wide application prospects, and simplifying the operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
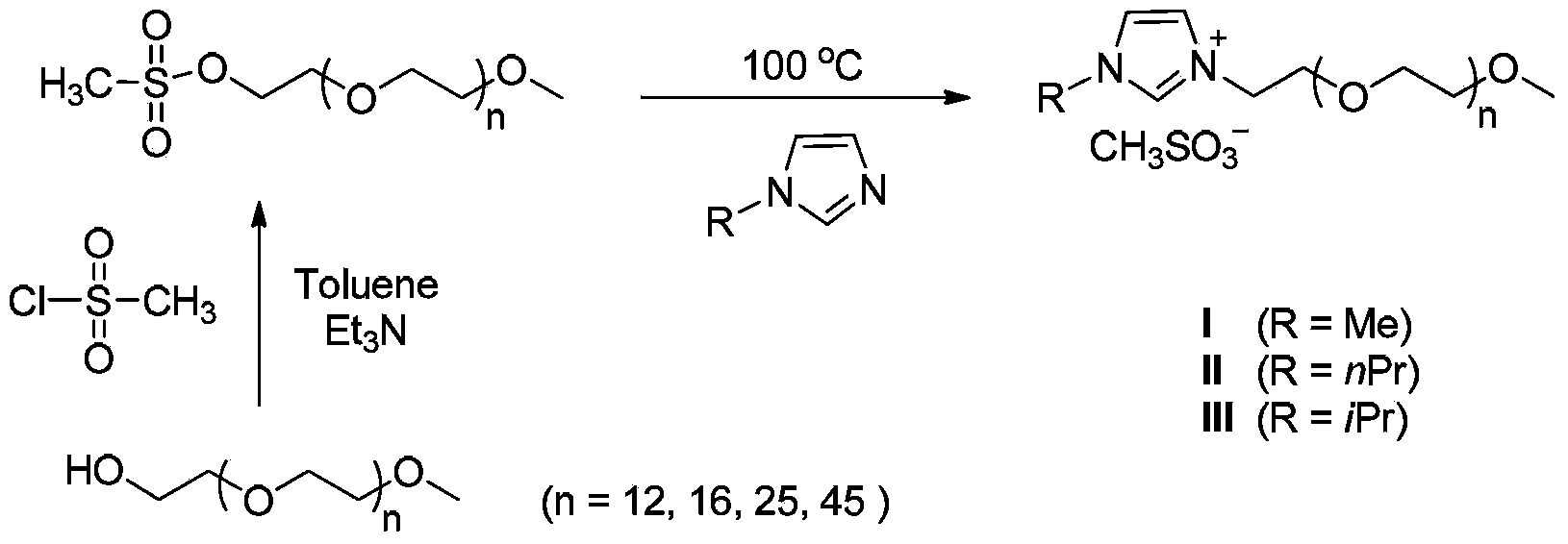
[0015] The preparation of embodiment 1 4-phenyl anisole
[0016] First, palladium chloride (0.0025mmol), imidazolium salt I (0.01mmol), 4-bromoanisole (0.5mmol), phenylboronic acid (0.75mmol), triethylamine (1.0mmol) and water (1mL) were added successively. into a round-bottomed flask and react for 10 minutes at 100°C with magnetic stirring. After the reaction was completed, 15 mL of saturated brine was added to quench the reaction, and the reaction mixture was extracted with ethyl acetate (15 mL×3). The eluent used for analysis is petroleum ether, and the product structure is passed through 1 H NMR and mass spectral identification. The separation yield reached 97%.
Embodiment 2
[0017] The preparation of embodiment 2 4-phenylnitrobenzene
[0018] First, palladium acetate (0.0025mmol), imidazolium salt II (0.01mmol), 4-bromonitrobenzene (0.5mmol), phenylboronic acid (0.75mmol), triethylamine (1.0mmol) and water (1mL) were added to In a round bottom flask, react under magnetic stirring at 100°C for 10 minutes. After the reaction was completed, 15 mL of saturated brine was added to quench the reaction, and the reaction mixture was extracted with ethyl acetate (15 mL×3). The eluent used for analysis is petroleum ether, and the product structure is passed through 1 H NMR and mass spectral identification. The separation yield reached 96%.
Embodiment 3
[0019] The preparation of embodiment 3 4-phenylacetophenone
[0020] First, palladium chloride (0.0025mmol), imidazolium salt III (0.01mmol), 4-bromoacetophenone (0.5mmol), phenylboronic acid (0.75mmol), triethylamine (1.0mmol) and water (1mL) were added in sequence. into a round bottom flask and react for 5 minutes at 100°C with magnetic stirring. After the reaction was completed, 15 mL of saturated brine was added to quench the reaction, and the reaction mixture was extracted with ethyl acetate (15 mL×3). The eluent used for analysis is petroleum ether, and the product structure is passed through 1 H NMR and mass spectral identification. The separation yield reaches 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com