Compound with novel structure and preparation method and applications thereof
A compound, hydrogen sulfide technology, applied in the field of medicine, to achieve the effect of reducing cardiovascular events, high pharmacological activity, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. Synthesis of 5-(4-hydroxyphenyl)-3H-1,2-dithiocyclopentyl-4-ene-3-thione (ADT-OH)
[0045] (1) 5-(4-methoxyphenyl)-3H-1,2-dithiole-3-thione (ADT-OCH 3 )Synthesis
[0046] Add anethole (3.17g, 0.021mol), sulfur (4.70g, 0.146mol) and DMF30mL into the flask, stir and reflux for 6h. Pour into cold water after the reaction, a large amount of precipitates precipitated out, filtered, and recrystallized from ethanol-water to obtain red crystals with a yield of 78%, mp: 110~111°C.
[0047] 1 H NMR (400MHz, DMSO-d 6 ), δ(ppm): 7.62 (d, 2H, J = 8.9 Hz, ArH), 7.40 (s, 1H, =CH), 6.98 (d, 2H, J = 8.8 Hz, ArH), 3.88 (s, 3H, OCH 3 ).
[0048] (2) Synthesis of 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione (ADT-OH)
[0049] ADT-OCH 3 (20.6g, 0.1mol) and pyridine hydrochloride (69.0g, 0.6mol) were heated to reflux for 1h, cooled, turned into a solid, smashed, washed several times with water, filtered, and the filter cake was recrystallized with acetone-water, the yield was...
Embodiment 2
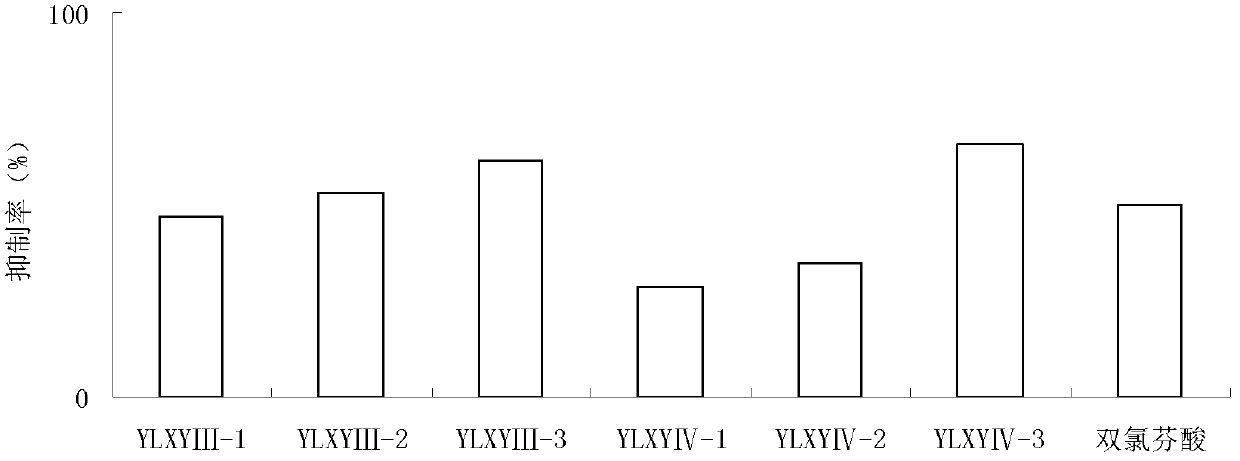
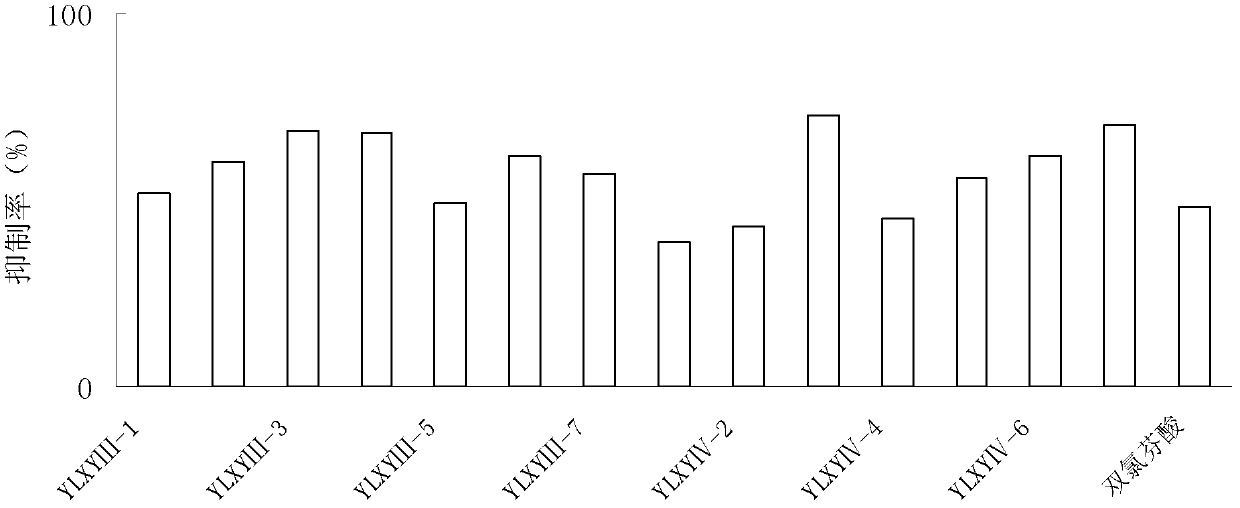
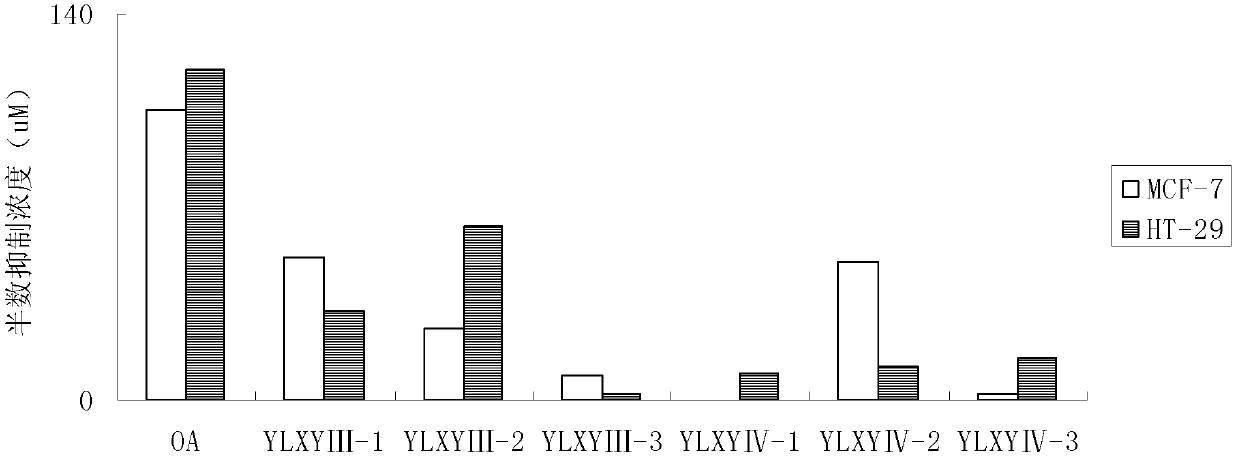
[0100] Synthesis of YLXY Ⅲ and Ⅳ Compounds
[0101] (1) Synthesis of oleanolic acid 2-[4-(3H-1, 2-dithiol-3-thione-5-yl)-phenoxy]ethyl ester (YLXYⅢ-1)
[0102] Will ADT-OH (0.147g, 0.65mmol), oleanolic acid-2-bromoethyl ester ( 9a ) (0.365 g, 0.65mmol), anhydrous K 2 CO 3 (0.178g, 1.30mmol) and a catalytic amount of KI were added to 10mL dry DMF, stirred at room temperature for 12h, then diluted with 10mL ethyl acetate, washed with water (3×10mL), anhydrous Na 2 SO 4 Dry, evaporate the solvent to dryness, and perform column chromatography [petroleum ether (60-90):ethyl acetate=20:1 (v / v)] to obtain 0.259 g of a red solid with a yield of 56.2%, mp: 101.0~102.0°C.
[0103] 1 H NMR (400 MHz, CDCl 3 ) , δ: 7.62 (d, 2H, J = 8.8 Hz, ArH), 7.39 (s, 1H, =CH), 6.97 (d, 2H, J = 8.8 Hz, ArH), 5.24 (brs, 1H, C 12 -H), 4.41 (m, 2H, OCH 2 ), 4.21 (t, 2H, OCH 2 ), 3.19 (dd, 1H, 3α-H), 2.86 (d, 1H, C 18 -H), 1.11 (s, 3H, CH 3 ), 0.97 (s, 3H, CH 3 ), 0.91 (s, 3H, CH 3 ), ...
Embodiment 3
[0140] (1) 3-[4-(3H-1, 2-dithiol-3-thione-5-yl)-phenoxy]propyl 3-oxooleanolic acid (YLXYⅣ-1 )Synthesis
[0141] Referring to the synthetic method of YLXYⅢ-1, 3-oxooleanolic acid and 3b Preparation, red powder, yield 82.6%, mp: 91.6~92.5℃.
[0142] 1 H NMR (400MHz, CDCl 3 ), δ(ppm): 7.62 (d, 2H, J = 8.7 Hz, ArH), 7.40 (s, 1H, =CH), 6.96 (d, 2H, J = 8.7 Hz, ArH), 5.26 (s, 1H, C 12 -H), 4.30-4.16 (m, 2H, OCH 2 ), 4.15-4.05 (m, 2H, OCH 2 ), 2.88 (m, 1H, C 18 -H), 2.57-2.47 (m, 1H, C 2 -H), 2.39-2.28 (m, 1H, C 2 -H), 2.15 (p, 2H, CH 2 ), 1.10 (s, 3H, CH 3 ), 1.05 (s, 3H, CH 3 ), 1.00 (s, 3H, CH 3 ), 0.93 (s, 3H, CH 3 ), 0.91 (s, 3H, CH 3 ), 0.89 (s, 3H, CH 3 ), 0.66 (s, 3H, CH 3 ).
[0143] IR(KBr, cm -1 ): 1716.7 (C=O), 1701.0 (C=O), 1662.4, 1601.0, 1576.8, 1490.3 (C=C), 1177.5 (C=S).
[0144] HR-MS: Calcd. For C 42 h 57 o 4 S 3 [M+H]+ 721.3413, Found: 721.3413.
[0145] (2) 4-[4-(3H-1,2-dithiol-3-thione-5-yl)-phenoxy]butyl 3-oxooleanolic acid (YLXYⅣ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com