Stable peroxyacetic acid solution
A technology of peracetic acid and stabilizer, applied in the field of peracetic acid solution and preparation thereof, can solve problems such as being unsuitable for bleaching, and achieve the effects of insufficient stability, few additions, and simple formula
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The stabilizer of the present embodiment, it comprises A component and B component, wherein:
[0037] The composition of A component is: industrial product aminotrimethylene phosphonic acid aqueous solution (wherein, aminotrimethylene phosphonic acid content is 50wt%, pyrophosphoric acid, phosphoric acid, phosphorous acid etc. phosphoric acid equivalent content 2%);
[0038] The composition of component B is: 10wt% sodium hydroxide, 25ppm sodium pyridinedicarboxylate, 25ppm sodium edetate, 25ppm sodium citrate, 25ppm sodium silicate, and 90wt% water;
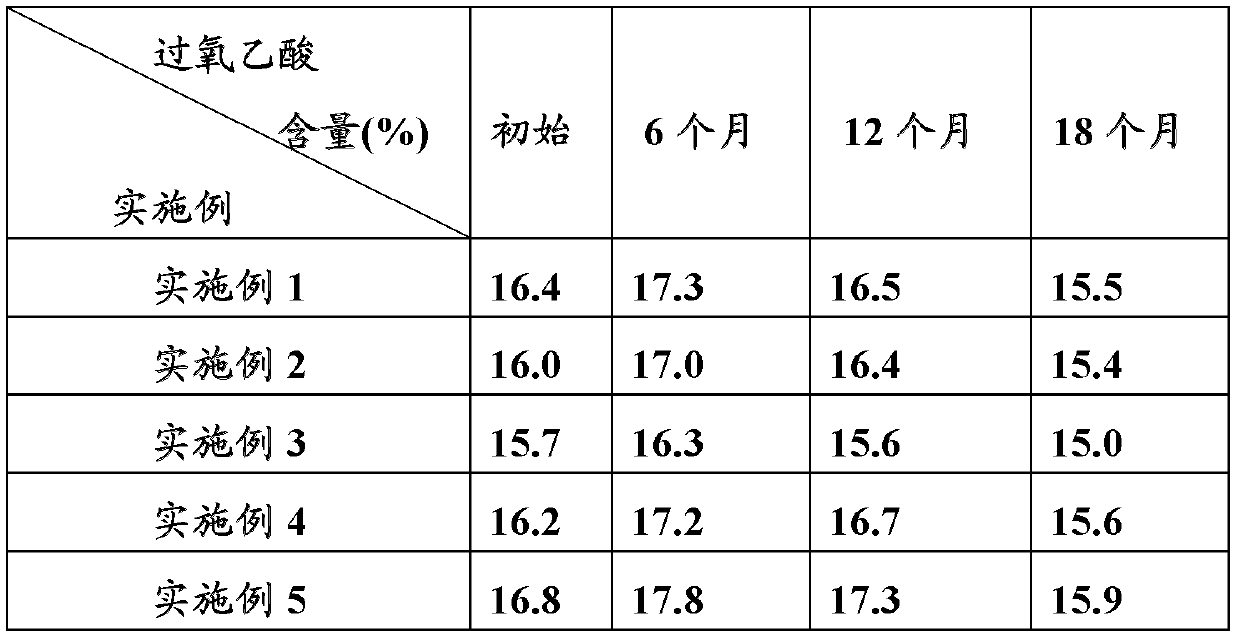
[0039]Make peracetic acid solution finished product according to above-mentioned method, after testing, peracetic acid solution finished product main component is as follows: peracetic acid 16.4%, hydrogen peroxide 21.5%, burn content 0.045%, sulfate, arsenic, lead are qualified.
Embodiment 2
[0041] The stabilizer of the present embodiment, it comprises A component and B component, wherein:
[0042] The composition of A component is: industrial product aminotrimethylene phosphonic acid aqueous solution (wherein, aminotrimethylene phosphonic acid content is 50wt%, pyrophosphoric acid, phosphoric acid, phosphorous acid etc. phosphoric acid equivalent content 3%);
[0043] The composition of component B is: sodium hydroxide 15wt%, sodium pyridinedicarboxylate 25ppm, sodium edetate 25ppm, sodium citrate 10ppm, sodium silicate 15ppm, water 85wt%;
[0044] According to above-mentioned method, make peracetic acid solution finished product, after testing, peracetic acid solution finished product main component is as follows: peracetic acid 16.0%, hydrogen peroxide 21.6%, burn content 0.048%, sulfate, arsenic, lead are qualified.
Embodiment 3
[0046] The stabilizer of the present embodiment, it comprises A component and B component, wherein:
[0047] The composition of A component is: industrial product diethylenetriaminepentamethylenephosphonic acid aqueous solution (wherein, the content of diethylenetriaminepentamethylenephosphonic acid is 50wt%, and the equivalent content of phosphoric acid such as pyrophosphoric acid, phosphoric acid and phosphorous acid is 3 %);
[0048] The composition of component B is: sodium hydroxide 12.5wt%, sodium pyridinedicarboxylate 20ppm, sodium edetate 10ppm, sodium citrate 10ppm, sodium silicate 10ppm, water 87.5wt%;
[0049] Make peracetic acid solution finished product according to above-mentioned method, after testing, peracetic acid solution finished product main component is as follows: peracetic acid 15.7%, hydrogen peroxide 21.4%, burn content 0.043%, sulfate, arsenic, lead are qualified.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com