Mid-infrared luminescent chalcohalide glass and preparation technology thereof
A technology of sulfur halide glass and infrared luminescence, which is applied in the field of optical materials, can solve the problems of reducing the efficiency of infrared luminescence, and achieve the effect of simple preparation process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0013] Example 1: Ge (99.999%), Ga (99.99%), S (99.999%), CsCl (99.9%), Nd 2 S 3 (99.9%) and Er 2 S 3 (99.9%) high-purity raw materials according to 65GeS 2 -24.7Ga 2 S 3 -10CsCl-0.25Er 2 S 3 -0.05Nd 2 S 3 Weigh the molar components of the molar components, mix them evenly, and put them into a quartz glass tube; connect the open end of the quartz tube to a vacuum system, and pump out the water and air in the quartz tube and the medicine; when the vacuum reaches 10 -2 At Pa, seal the quartz tube with an oxyhydrogen flame; put the sealed quartz tube into a swing furnace, heat up to 900°C and start to swing, and keep it warm for 12 hours to melt it; then, take out the quartz tube and place it vertically Quenched in water for a few seconds to obtain a block of sulfur halide glass. The sulfur halide glass is annealed in a resistance furnace at 280°C to eliminate internal stress.
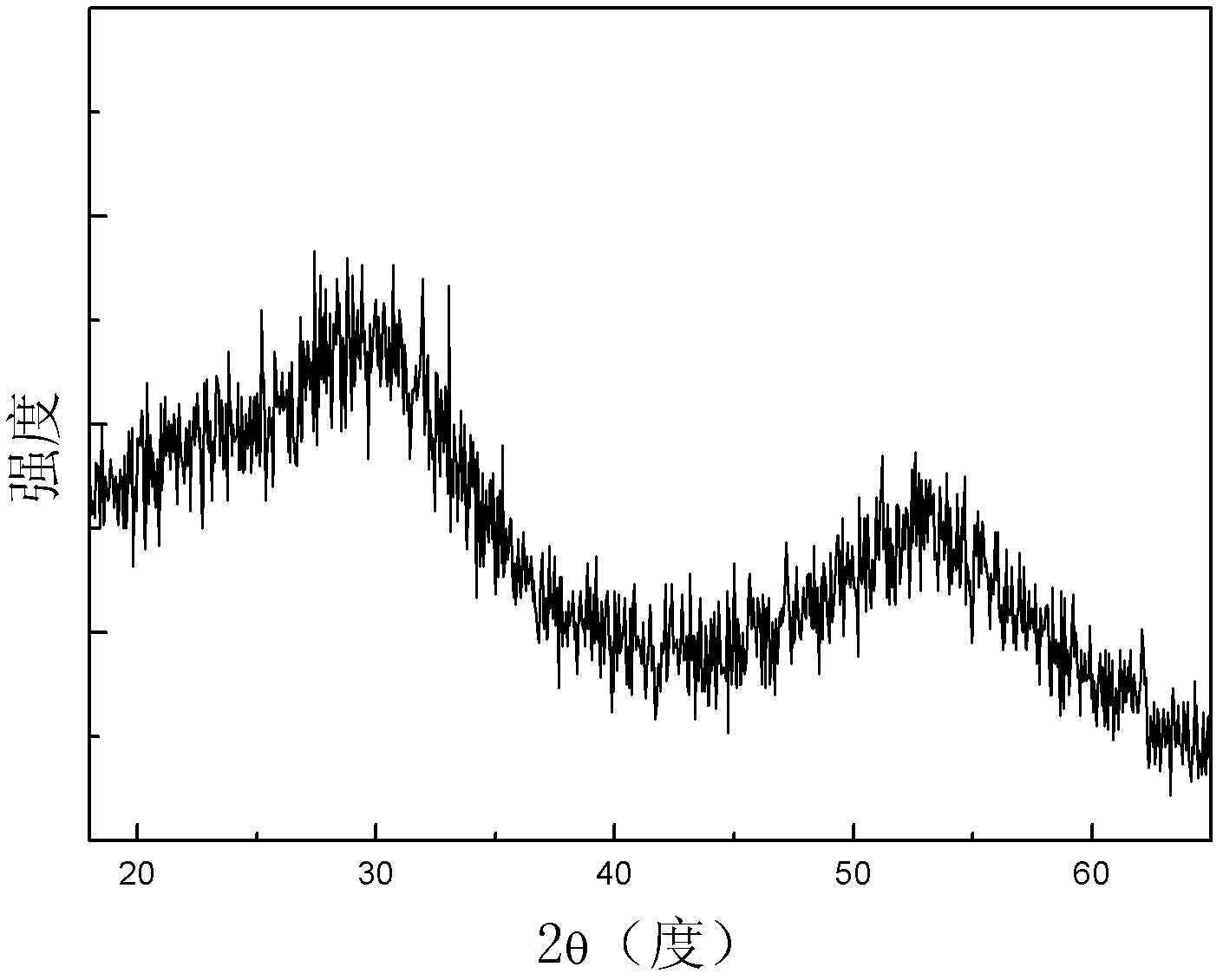
[0014] Powder X-ray Diffraction Pattern ( figure 1 ) analysis shows that the prepared sulf...
example 2
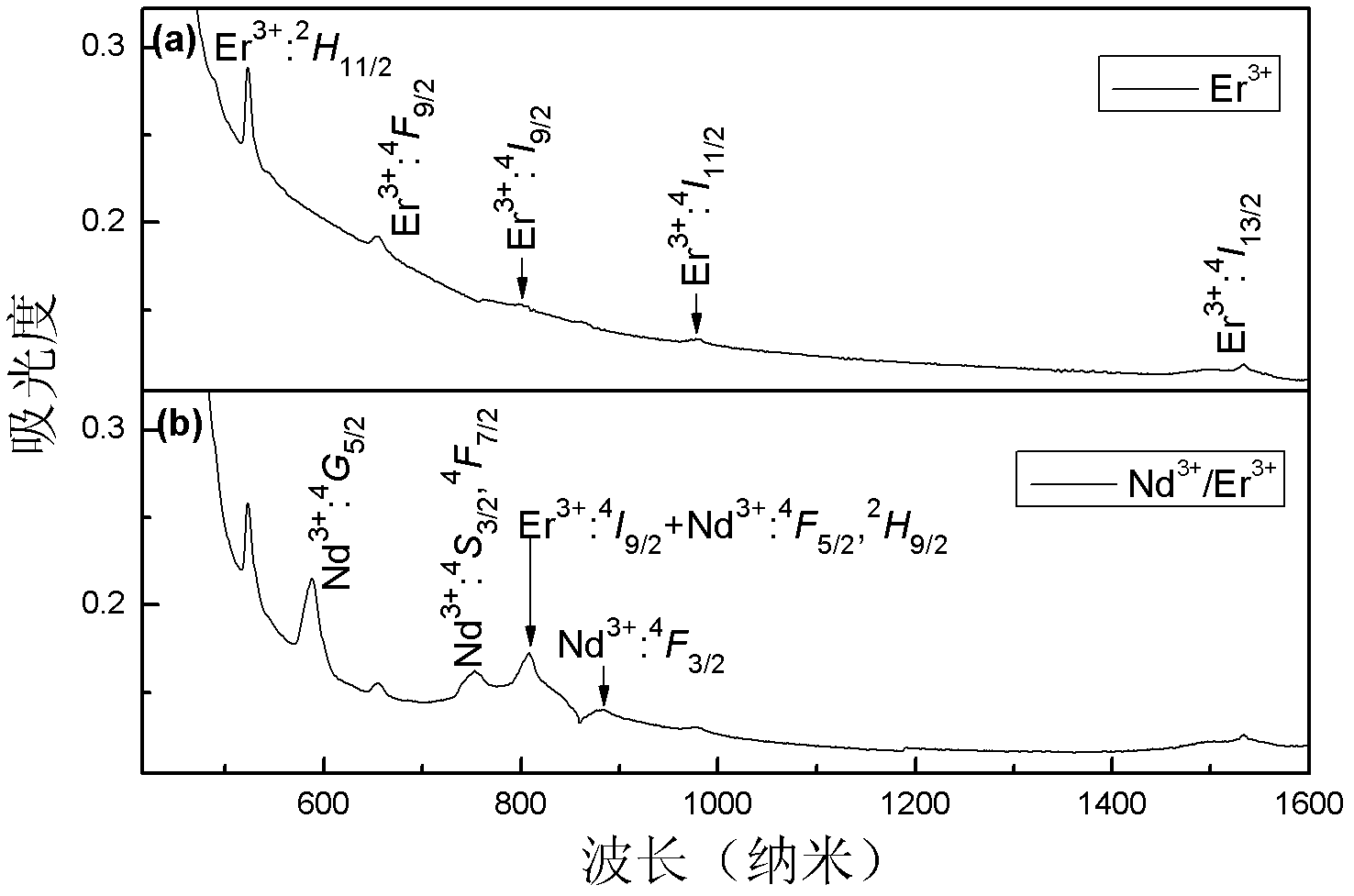
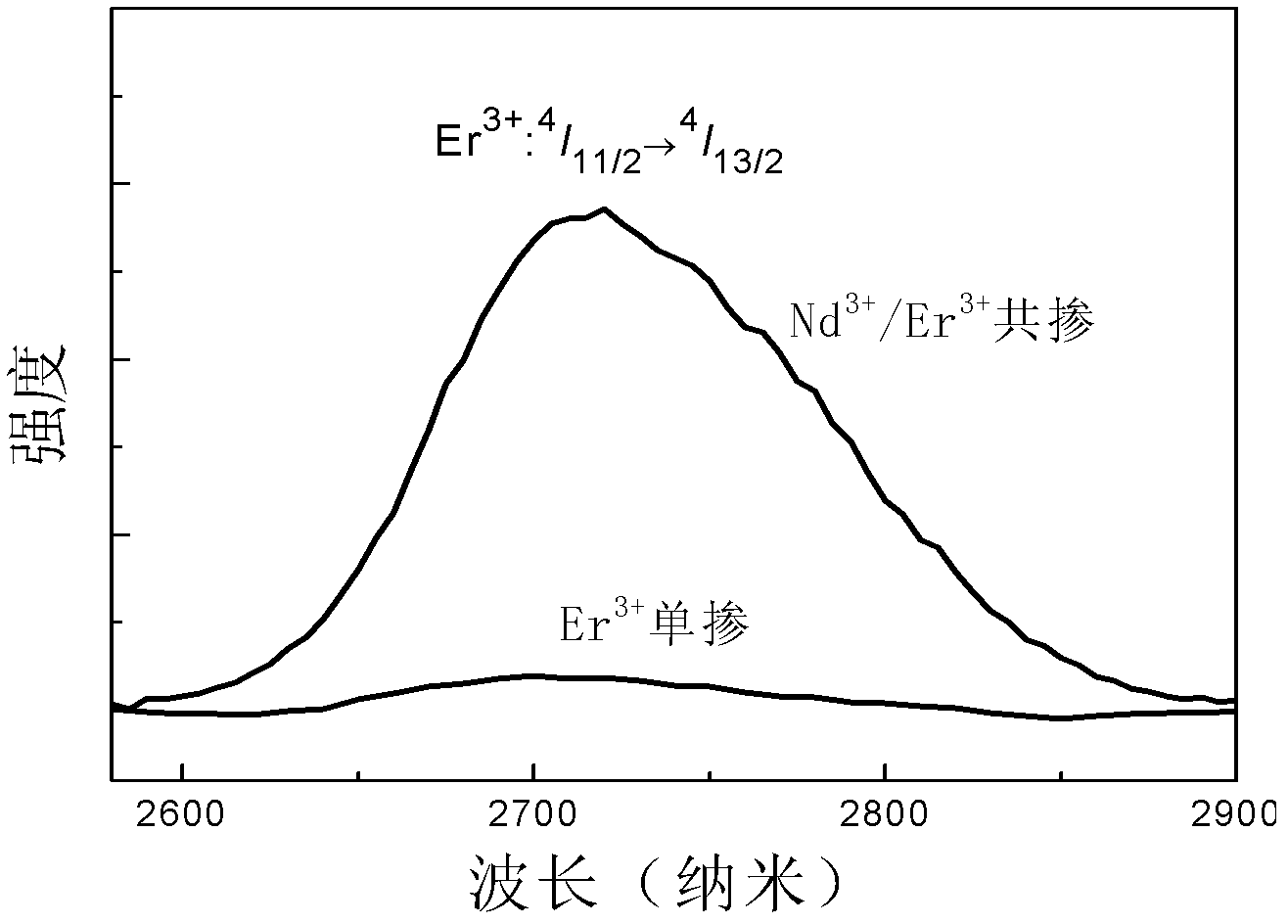
[0015] Example 2: Ge (99.999%), Ga (99.99%), S (99.999%), CsCl (99.9%), Nd 2 S 3 (99.9%) and Er 2 S 3 (99.9%) high-purity raw materials according to 55GeS 2 -34.7Ga 2 S 3 -10CsCl-0.15Er 2 S 3 -0.15 Nd 2 S 3 After the molar composition ratio of the molar composition is weighed, put it into a quartz glass tube; connect the open end of the quartz tube to a vacuum system, and pump out the water and air in the quartz tube and the medicine; when the vacuum degree reaches 10 -2 At Pa, seal the quartz tube with an oxyhydrogen flame; put the sealed quartz tube into a swing furnace, heat up to 1000°C and start to swing, and keep it warm for 18 hours to melt it; then, take out the quartz tube and place it vertically Quenched in water for a few seconds to obtain a block of sulfur halide glass. The sulfur halide glass is annealed in a resistance furnace at 280°C to eliminate internal stress. After the sample is surface polished, use the FSP920 spectrophotometer equipped with an ...
example 3
[0016] Example 3: Ge (99.999%), Ga (99.99%), S (99.999%), CsCl (99.9%), Nd 2 S 3 (99.9%) and Er 2 S 3 (99.9%) high-purity raw materials according to 50GeS 2 -34.7Ga 2 S 3 -15CsCl-0.1Er 2 S 3 -0.2Nd 2 S 3 After the molar composition ratio of the molar composition is weighed, put it into a quartz glass tube; connect the open end of the quartz tube to a vacuum system, and pump out the water and air in the quartz tube and the medicine; when the vacuum degree reaches 10 -2At Pa, seal the quartz tube with an oxyhydrogen flame; put the sealed quartz tube into a swing furnace, raise the temperature to 950°C, start to swing, and keep it warm for 24 hours to melt it; then, take out the quartz tube and place it vertically Quenched in water for a few seconds to obtain a block of sulfur halide glass. The sulfur halide glass is annealed in a resistance furnace at 280°C to eliminate internal stress. After the sample is surface polished, use the FSP920 spectrophotometer equipped wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com